Arch Hand Microsurg.
2022 Dec;27(4):345-353. 10.12790/ahm.22.0056.
Resurfacing defects from mycobacterial skin and soft tissue infections using thoracodorsal artery perforator free flaps
- Affiliations
-
- 1Department of Plastic and Reconstructive Surgery, Hanyang University College of Medicine, Seoul, Korea
- KMID: 2536223
- DOI: http://doi.org/10.12790/ahm.22.0056
Abstract
- Purpose
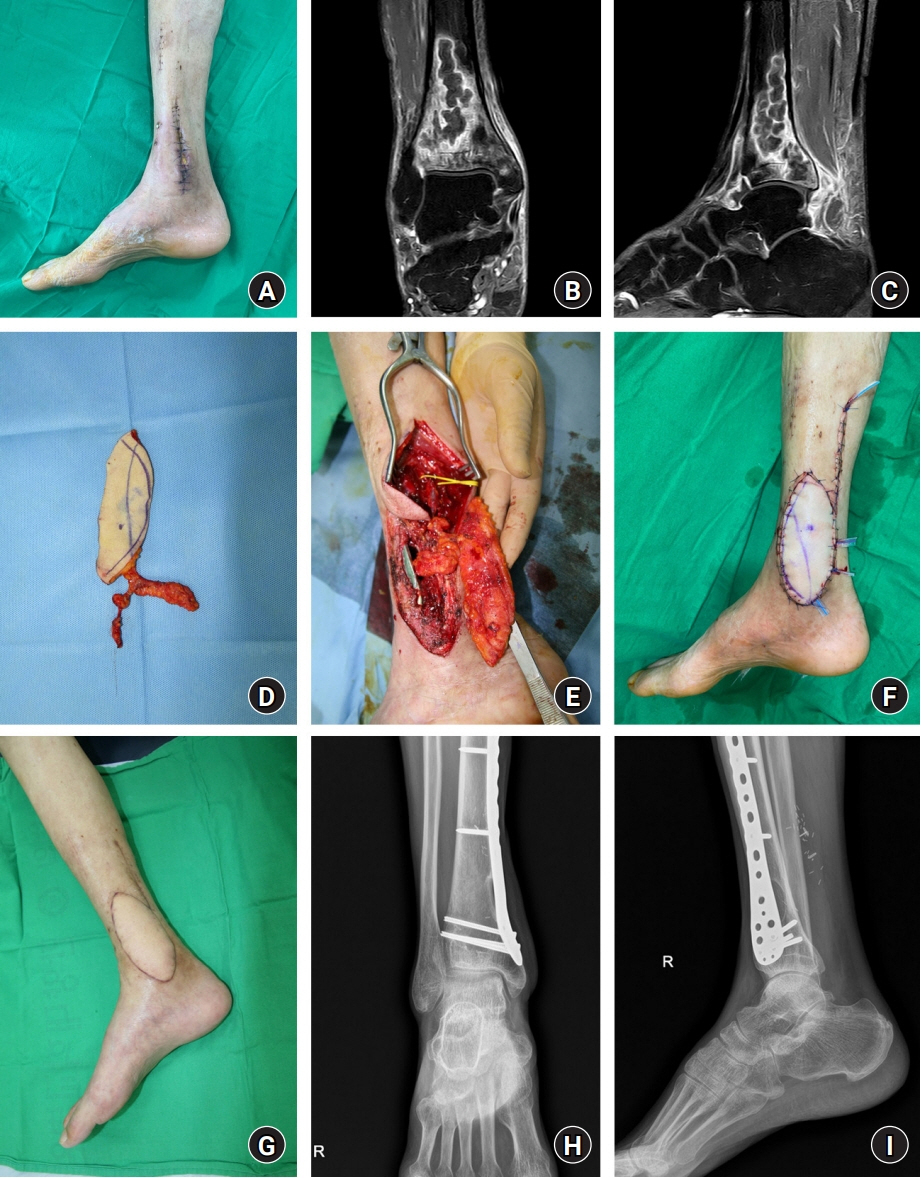
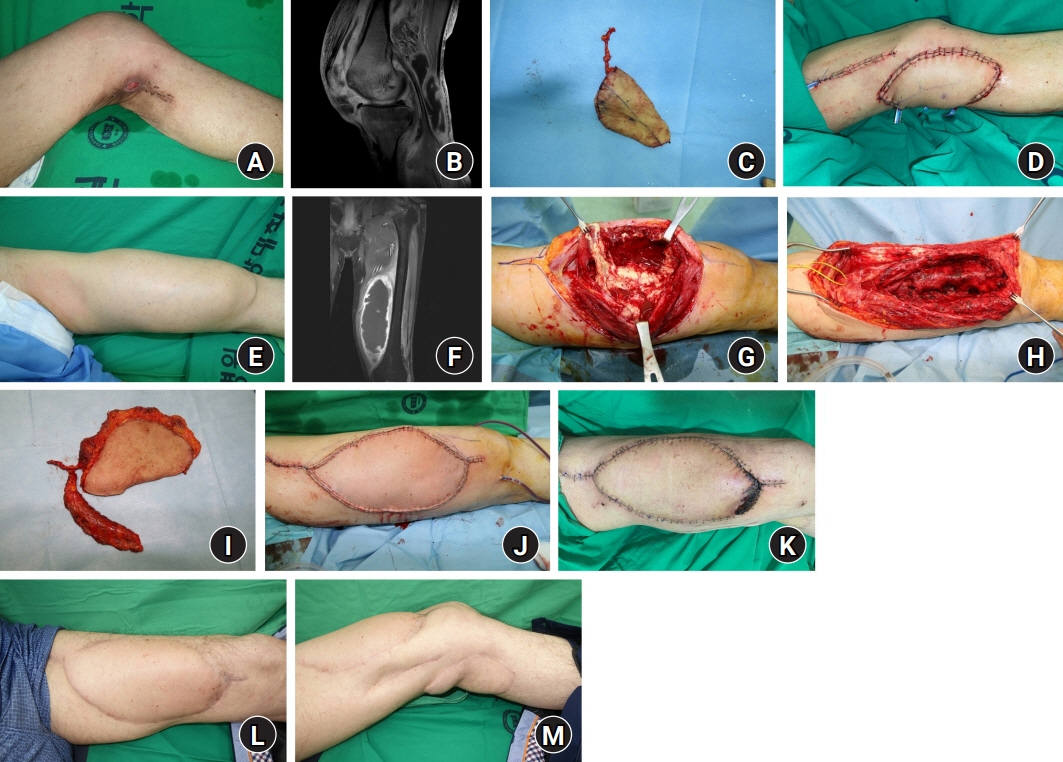
Skin and soft tissue infections (SSTIs) caused by mycobacteria are rare and difficult to diagnose and treat. Furthermore, systematic treatment protocols for mycobacterial SSTIs have not been established. This study introduces a strategy with radical resection and resurfacing using thoracodorsal artery perforator (TDAP) free flaps.
Methods
From December 2013 to February 2022, 13 patients with mycobacterial SSTIs underwent radical resection and reconstruction using TDAP free flaps. Exact mapping of the lesion extent was performed preoperatively with magnetic resonance imaging. When the extent was limited to soft tissue, resection and reconstruction were performed in a single stage. However, in cases with bone or joint involvement, two-stage reconstruction was applied with radical resection and negative-pressure wound therapy followed by resurfacing with a flap. Complex defects formed after resection were filled with a musculocutaneous or chimeric flap. All patients were administered antimycobacterial medications.
Results
Mycobacterial infection recurred in one patient; therefore, a total of 14 cases of reconstruction were performed. Reconstruction was performed with a TDAP free flap alone in 10 cases, with a chimeric flap in three cases, and with a musculocutaneous flap in one case. The flaps ranged in size from 7×5 cm2 to 25×12 cm2 (mean, 97.2 cm2). The mycobacterial species identified were Mycobacterium tuberculosis (n=8) and nontuberculous mycobacteria (n=5).
Conclusion
For mycobacterial SSTIs, radical resection followed by resurfacing and reconstruction using TDAP free flaps can be an effective surgical treatment strategy.
Figure
Reference
-
References
1. Franco-Paredes C, Marcos LA, Henao-Martínez AF, et al. Cutaneous mycobacterial infections. Clin Microbiol Rev. 2018; 32:e00069–18.
Article2. Wang SH, Pancholi P. Mycobacterial skin and soft tissue infection. Curr Infect Dis Rep. 2014; 16:438.
Article3. Nogueira LB, Garcia CN, Costa MS, Moraes MB, Kurizky PS, Gomes CM. Non-tuberculous cutaneous mycobacterioses. An Bras Dermatol. 2021; 96:527–38.
Article4. Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007; 175:367–416.5. Bae JY, Yun IS, Roh TS, Kim YS. Treatment strategy for skin and soft tissue infections caused by nontuberculous mycobacteria following various procedures. Arch Aesthetic Plast Surg. 2021; 27:3–11.
Article6. Bhambri S, Bhambri A, Del Rosso JQ. Atypical mycobacterial cutaneous infections. Dermatol Clin. 2009; 27:63–73.
Article7. Lamb RC, Dawn G. Cutaneous non-tuberculous mycobacterial infections. Int J Dermatol. 2014; 53:1197–204.
Article8. van Mechelen M, van der Hilst J, Gyssens IC, Messiaen P. Mycobacterial skin and soft tissue infections: TB or not TB? Neth J Med. 2018; 76:269–74.9. Gonzalez-Santiago TM, Drage LA. Nontuberculous mycobacteria: skin and soft tissue infections. Dermatol Clin. 2015; 33:563–77.10. Koschnick M, Bruener S, Germann G. Free tissue transfer: an advanced strategy for postinfection soft-tissue defects in the upper extremity. Ann Plast Surg. 2003; 51:147–54.
Article11. Kim SH, Lee JH, Kim SE, et al. Retrospective study of the efficacy of vascularized tissue transfer for treating antibiotic-resistant bacteria-infected wound: comparison with clean and antibiotic-sensitive bacteria-infected wound. Medicine (Baltimore). 2021; 100:e25907.12. Yu JL, Crowe CS, Yesantharao P, Kennedy SA, Keys KA. Soft tissue reconstruction for upper extremity necrotizing soft tissue infections. Ann Plast Surg. 2022; 89:631–6.
Article13. World Health Organization WHO). Global tuberculosis report 2021 [Internet]. Geneva: WHO;2021. [cited 2022 Oct 9]. Available from: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2021.14. Khan FA, Khakoo R. Nontuberculous mycobacterial cutaneous infections: an updated review. Cutis. 2011; 88:194–200.15. Lim JM, Kim JH, Yang HJ. Management of infections with rapidly growing mycobacteria after unexpected complications of skin and subcutaneous surgical procedures. Arch Plast Surg. 2012; 39:18–24.
Article16. Gao LJ, Huang ZH, Jin QY, et al. Delayed diagnosis and comprehensive treatment of cutaneous tuberculosis: a case report. World J Clin Cases. 2021; 9:4007–15.
Article17. Kim HR, Yoon ES, Kim DW, et al. Empirical treatment of highly suspected nontuberculous mycobacteria infections following aesthetic procedures. Arch Plast Surg. 2014; 41:759–67.
Article18. Saeed A, Narayan N, Karmiris N, Troisi L. Mycobacterium szulgai infection after carpal tunnel release requiring extensive debridement and ALT flap reconstruction. J Hand Microsurg. 2020; 13:252–4.
Article19. Kim SW, Hwang KT, Kim JD, Kim YH. Reconstruction of postinfected scalp defects using latissimus dorsi perforator and myocutaneous free flaps. J Craniofac Surg. 2012; 23:1615–9.
Article20. Kim JT, Kim SW. Improvement of ischemic or congested wound conditions by reconstruction with microsurgical flaps. Microsurgery. 2018; 38:388–94.
Article21. Cedidi C, Raff T, Germann G. The radial forearm fascial flap as a salvage procedure for chronic tuberculosis of the hand and wrist. J Hand Surg Br. 1998; 23:476–8.
Article22. Orgill DP, Bayer LR. Update on negative-pressure wound therapy. Plast Reconstr Surg. 2011; 127 Suppl 1:105S–115S.
Article23. Gravvanis A, Tsoutsos D, Karakitsos D, Iconomou T, Papadopoulos O. Blood perfusion of the free anterolateral thigh perforator flap: its beneficial effect in the reconstruction of infected wounds in the lower extremity. World J Surg. 2007; 31:11–8.
Article24. Manoso MW, Boland PJ, Healey JH, Cordeiro PG. Limb salvage of infected knee reconstructions for cancer with staged revision and free tissue transfer. Ann Plast Surg. 2006; 56:532–5.
Article25. Kim JT, Kim SW, Youn S, Kim YH. What is the ideal free flap for soft tissue reconstruction?: a ten-year experience of microsurgical reconstruction using 334 latissimus dorsi flaps from a universal donor site. Ann Plast Surg. 2015; 75:49–54.
Article26. Ayhan S, Tuncer S, Demir Y, Kandal S. Thoracodorsal artery perforator flap: a versatile alternative for various soft tissue defects. J Reconstr Microsurg. 2008; 24:285–93.
Article27. Guerra AB, Metzinger SE, Lund KM, Cooper MM, Allen RJ, Dupin CL. The thoracodorsal artery perforator flap: clinical experience and anatomic study with emphasis on harvest techniques. Plast Reconstr Surg. 2004; 114:32–43.
Article28. Wei FC, Mardini S. Free-style free flaps. Plast Reconstr Surg. 2004; 114:910–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Reconstruction of the Soft Tissue Defect Using Thoracodorsal Artery Perforator Skin Flap
- Reconstruction of a Mangled Hand with a Thoracodorsal Artery Perforator Free Flap: A Report of Two Cases
- Reconstruction of Greater Trochanteric defect using Lumbar Artery Perforator Free Flap: A Case Report
- Lower Extremity Reconstruction of Soft Tissue Defects with Perforator Island Flap
- Soft Tissue Reconstruction of Children's Extremity with Perforator free Flap