J Korean Neurosurg Soc.
2022 Nov;65(6):790-800. 10.3340/jkns.2021.0298.
EID3 Promotes Glioma Cell Proliferation and Survival by Inactivating AMPKα1
- Affiliations
-
- 1Department of Hand Surgery, Huashan Hospital, Fudan University, Shanghai, China
- 2NHC Key Laboratory of Hand Reconstruction (Fudan University), Ministry of Health, Shanghai, China
- 3Shanghai Key Laboratory of Peripheral Nerve and Microsurgery, Shanghai, China
- 4Department of Radiology, Beijing Electric Power Hospital, Beijng, China
- 5Department of Neurosurgery, Minhang Hospital, Fudan University, Shanghai, China
- 6School of Rehabilitation Science, Shanghai University of Traditional Chinese Medicine, Shanghai, China
- KMID: 2535839
- DOI: http://doi.org/10.3340/jkns.2021.0298
Abstract
Objective
: EID3 (EP300-interacting inhibitor of differentiation) was identified as a novel member of EID family and plays a pivotal role in colorectal cancer development. However, its role in glioma remained elusive. In current study, we identified EID3 as a novel oncogenic molecule in human glioma and is critical for glioma cell survival, proliferation and invasion.
Methods
: A total of five patients with glioma were recruited in present study and fresh glioma samples were removed from patients. Four weeks old male non-obese diabetic severe combined immune deficiency (NOD/SCID) mice were used as transplant recipient models. The subcutaneous tumor size was calculated and recorded every week with vernier caliper. EID3 and AMP-activated protein kinase α1 (AMPKα1) expression levels were confirmed by real-time polymerase chain reaction and Western blot assays. Colony formation assays were performed to evaluate cell proliferation. Methyl thiazolyl tetrazolium (MTT) assays were performed for cell viability assessment. Trypan blue staining approach was applied for cell death assessment. Cell Apoptosis DNA ELISA Detection Kit was used for apoptosis assessment.
Results
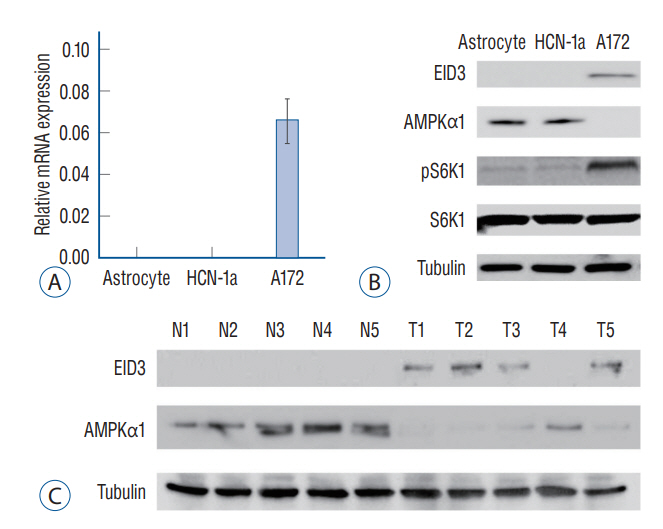
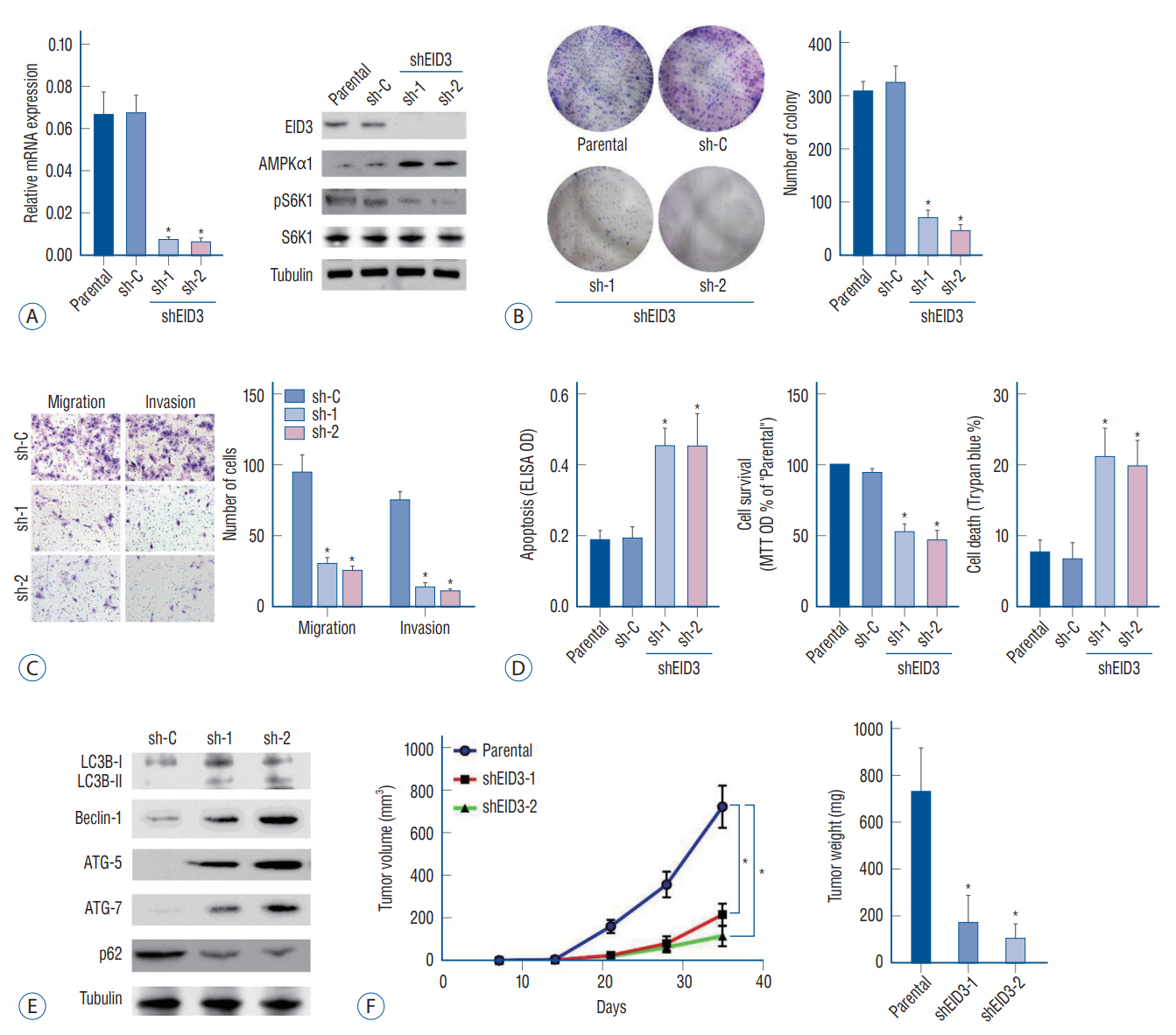
: EID3 was preferentially expressed in glioma tissues/cells, while undetectable in astrocytes, neuronal cells, or normal brain tissues. EID3 knocking down significantly hindered glioma cell proliferation and invasion, as well as induced reduction of cell viability, apoptosis and cell death. EID3 knocking down also greatly inhibited tumor growth in SCID mice. Knocking down of AMPKα1 could effectively rescue glioma cells from apoptosis and cell death caused by EID3 absence, indicating that AMPKα1 acted as a key downstream regulator of EID3 and mediated suppression effects caused by EID3 knocking down inhibition. These findings were confirmed in glioma cells generated patient-derived xenograft models. AMPKα1 protein levels were affected by MG132 treatment in glioma, which suggested EID3 might down regulate AMPKα1 through protein degradation.
Conclusion
: Collectively, our study demonstrated that EID3 promoted glioma cell proliferation and survival by inhibiting AMPKα1 expression. Targeting EID3 might represent a promising strategy for treating glioma.
Figure
Reference
-
References
1. Almiron Bonnin DA, Havrda MC, Israel MA. Glioma cell secretion: a driver of tumor progression and a potential therapeutic target. Cancer Res. 78:6031–6039. 2018.2. Barthel FP, Wesseling P, Verhaak RGW. Reconstructing the molecular life history of gliomas. Acta Neuropathol. 135:649–670. 2018.3. Båvner A, Matthews J, Sanyal S, Gustafsson JA, Treuter E. EID3 is a novel EID family member and an inhibitor of CBP-dependent co-activation. Nucleic Acids Res. 33:3561–3569. 2005.4. Bilanges B, Alliouachene S, Pearce W, Morelli D, Szabadkai G, Chung YL, et al. Vps34 PI 3-kinase inactivation enhances insulin sensitivity through reprogramming of mitochondrial metabolism. Nat Commun. 8:1804. 2017.5. Boussiotis VA, Charest A. Immunotherapies for malignant glioma. Oncogene. 37:1121–1141. 2018.6. Cai S, Li Y, Bai JY, Zhang ZQ, Wang Y, Qiao YB, et al. Gαi3 nuclear translocation causes irradiation resistance in human glioma cells. Oncotarget. 8:35061–35068. 2017.7. Carling D. AMPK signalling in health and disease. Curr Opin Cell Biol. 45:31–37. 2017.8. Carroll B, Dunlop EA. The lysosome: a crucial hub for AMPK and mTORC1 signalling. Biochem J. 474:1453–1466. 2017.9. Chou CC, Lee KH, Lai IL, Wang D, Mo X, Kulp SK, et al. AMPK reverses the mesenchymal phenotype of cancer cells by targeting the AktMDM2-Foxo3a signaling axis. Cancer Res. 74:4783–4795. 2014.10. Cork GK, Thompson J, Slawson C. Real talk: the inter-play between the mTOR, AMPK, and hexosamine biosynthetic pathways in cell signaling. Front Endocrinol (Lausanne). 9:522. 2018.11. Cui Y, Zhao J, Yi L, Jiang Y. microRNA-153 targets mTORC2 component rictor to inhibit glioma cells. PLoS One. 11:e0156915. 2016.12. Dunlop EA, Tee AR. The kinase triad, AMPK, mTORC1 and ULK1, maintains energy and nutrient homoeostasis. Biochem Soc Trans. 41:939–943. 2013.13. Fu L, Zhang S, Zhang L, Tong X, Zhang J, Zhang Y, et al. Systems biology network-based discovery of a small molecule activator BL-AD008 targeting AMPK/ZIPK and inducing apoptosis in cervical cancer. Oncotarget. 6:8071–8088. 2015.14. Ghinda DC, Wu JS, Duncan NW, Northoff G. How much is enough-can resting state fMRI provide a demarcation for neurosurgical resection in glioma? Neurosci Biobehav Rev. 84:245–261. 2018.15. Guerineau M, Kriz Z, Kozakova L, Bednarova K, Janos P, Palecek J. Analysis of the Nse3/MAGE-binding domain of the Nse4/EID family proteins. PLoS One. 7:e35813. 2012.16. He L, Zhou X, Huang N, Li H, Tian J, Li T, et al. AMPK regulation of glucose, lipid and protein metabolism: mechanisms and nutritional significance. Curr Protein Pept Sci. 18:562–570. 2017.17. He S, Hu B, Li C, Lin P, Tang WG, Sun YF, et al. PDXliver: a database of liver cancer patient derived xenograft mouse models. BMC Cancer. 18:550. 2018.18. Hombach-Klonisch S, Mehrpour M, Shojaei S, Harlos C, Pitz M, Hamai A, et al. Glioblastoma and chemoresistance to alkylating agents: involvement of apoptosis, autophagy, and unfolded protein response. Pharmacol Ther. 184:13–41. 2018.19. Hsieh FS, Chen YL, Hung MH, Chu PY, Tsai MH, Chen LJ, et al. Palbociclib induces activation of AMPK and inhibits hepatocellular carcinoma in a CDK4/6-independent manner. Mol Oncol. 11:1035–1049. 2017.20. Jeon SM. Regulation and function of AMPK in physiology and diseases. Exp Mol Med. 48:e245. 2016.21. Jiang H, Liu W, Zhan SK, Pan YX, Bian LG, Sun B, et al. GSK621 targets glioma cells via activating AMP-activated protein kinase signalings. PLoS One. 11:e0161017. 2016.22. Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 13:132–141. 2011.23. Konagaya Y, Terai K, Hirao Y, Takakura K, Imajo M, Kamioka Y, et al. A highly sensitive FRET biosensor for AMPK exhibits heterogeneous AMPK responses among cells and organs. Cell Rep. 21:2628–2638. 2017.24. Lapointe S, Perry A, Butowski NA. Primary brain tumours in adults. Lancet. 392:432–446. 2018.25. Liu X, Hu X, Kuang Y, Yan P, Li L, Li C, et al. BCLB, methylated in hepatocellular carcinoma, is a starvation stress sensor that induces apoptosis and autophagy through the AMPK-mTOR signaling cascade. Cancer Lett. 395:63–71. 2017.26. Miller JJ, Shih HA, Andronesi OC, Cahill DP. Isocitrate dehydrogenase-mutant glioma: evolving clinical and therapeutic implications. Cancer. 123:4535–4546. 2017.27. Munakata K, Uemura M, Tanaka S, Kawai K, Kitahara T, Miyo M, et al. Cancer stem-like properties in colorectal cancer cells with low proteasome activity. Clin Cancer Res. 22:5277–5286. 2016.28. Neguembor MV, Xynos A, Onorati MC, Caccia R, Bortolanza S, Godio C, et al. FSHD muscular dystrophy region gene 1 binds suv4-20h1 histone methyltransferase and impairs myogenesis. J Mol Cell Biol. 5:294–307. 2013.29. O’Brien AJ, Villani LA, Broadfield LA, Houde VP, Galic S, Blandino G, et al. Salicylate activates AMPK and synergizes with metformin to reduce the survival of prostate and lung cancer cells ex vivo through inhibition of de novo lipogenesis. Biochem J. 469:177–187. 2015.30. Pan SJ, Ren J, Jiang H, Liu W, Hu LY, Pan YX, et al. MAGEA6 promotes human glioma cell survival via targeting AMPKα1. Cancer Lett. 412:21–29. 2018.31. Pei S, Minhajuddin M, Adane B, Khan N, Stevens BM, Mack SC, et al. AMPK/FIS1-mediated mitophagy is required for self-renewal of human AML stem cells. Cell Stem Cell. 23:86–100. 2018.32. Peng Z, Liu C, Wu M. New insights into long noncoding RNAs and their roles in glioma. Mol Cancer. 17:61. 2018.33. Quinones A, Le A. The multifaceted metabolism of glioblastoma. Adv Exp Med Biol. 1063:59–72. 2018.34. Sharples AP, Hughes DC, Deane CS, Saini A, Selman C, Stewart CE. Longevity and skeletal muscle mass: the role of IGF signalling, the sirtuins, dietary restriction and protein intake. Aging Cell. 14:511–523. 2015.35. Smith BK, Marcinko K, Desjardins EM, Lally JS, Ford RJ, Steinberg GR. Treatment of nonalcoholic fatty liver disease: role of AMPK. Am J Physiol Endocrinol Metab. 311:E730–E740. 2016.36. Umezawa S, Higurashi T, Nakajima A. AMPK: therapeutic target for diabetes and cancer prevention. Curr Pharm Des. 23:3629–3644. 2017.37. Wang C, Tong Y, Wen Y, Cai J, Guo H, Huang L, et al. Hepatocellular carcinoma-associated protein TD26 interacts and enhances sterol regulatory element-binding protein 1 activity to promote tumor cell proliferation and growth. Hepatology. 68:1833–1850. 2018.38. Weller M, Van den Bent M, Tonn JC, Stupp R, Preusser M, Cohen-Jonathan-Moyal E, et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 18:e315–e329. 2017.39. Xu L, Kong L, Wang J, Ash JD. Stimulation of AMPK prevents degeneration of photoreceptors and the retinal pigment epithelium. Proc Natl Acad Sci U S A. 115:10475–10480. 2018.40. Zhao W, Peng F, Shu M, Liu H, Hou X, Wang X, et al. Isogambogenic acid inhibits the growth of glioma through activation of the AMPK-mTOR pathway. Cell Physiol Biochem. 44:1381–1395. 2017.41. Zhao Y, Hu X, Liu Y, Dong S, Wen Z, He W, et al. ROS signaling under metabolic stress: cross-talk between AMPK and AKT pathway. Mol Cancer. 16:79. 2017.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Tetrandrine Exerts a Radiosensitization Effect on Human Glioma through Inhibiting Proliferation by Attenuating ERK Phosphorylation
- Effect of Tamoxifen in C6 Glioma Cells
- Long Noncoding RNA PVT1 Promotes Stemness and Temozolomide Resistance through miR-365/ELF4/SOX2 Axis in Glioma
- Myosin VI contributes to malignant proliferation of human glioma cells
- Effects of exogenous ATP on calcium mobilization and cell proliferation in C6 glioma cell