J Dent Rehabil Appl Sci.
2022 Jun;38(2):90-96. 10.14368/jdras.2022.38.2.90.
Effects of Shiitake mushroom extract on antimicrobial activity against periodontopathogens and inflammatory condition of human gingival fibroblast
- Affiliations
-
- 1Department of Preventive Dentistry, College of Dentistry, Dankook University, Cheonan, Republic of Korea
- KMID: 2535016
- DOI: http://doi.org/10.14368/jdras.2022.38.2.90
Abstract
- Purpose
The purpose of this study was to investigate antimicrobial activity of extracts from shiitake mushroom against periodontopathogens and its cytotoxicity for human gingival fibroblast.
Materials and Methods
Shiitake mushroom was soaked in water and acetone, and the supernatant was dried to collect its extract. The susceptibility of periodontopathogens for the extracts was investigated. Human gingival fibroblast was treated with the extracts, and the cell viability was measured CCK-8 solution.
Results
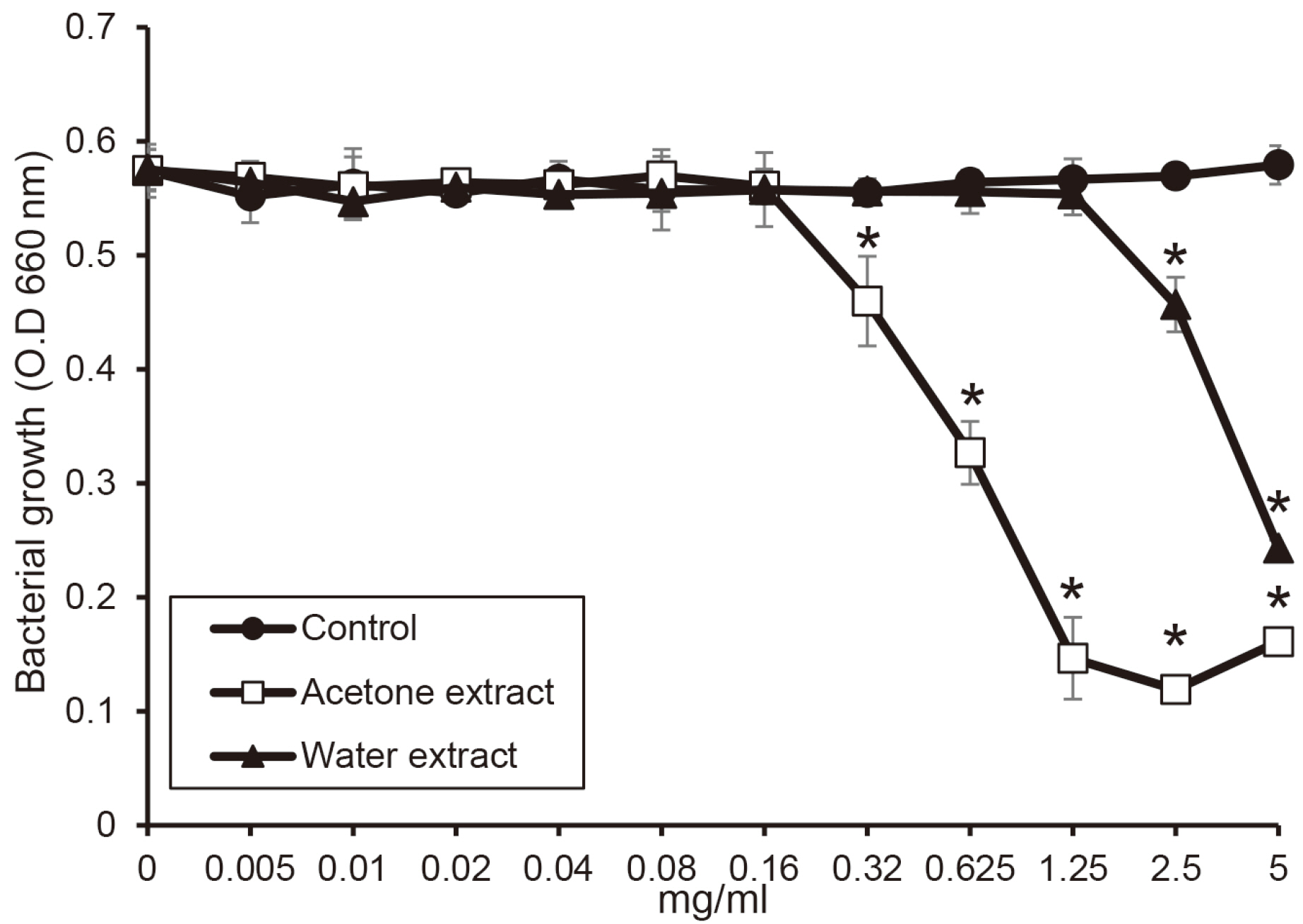
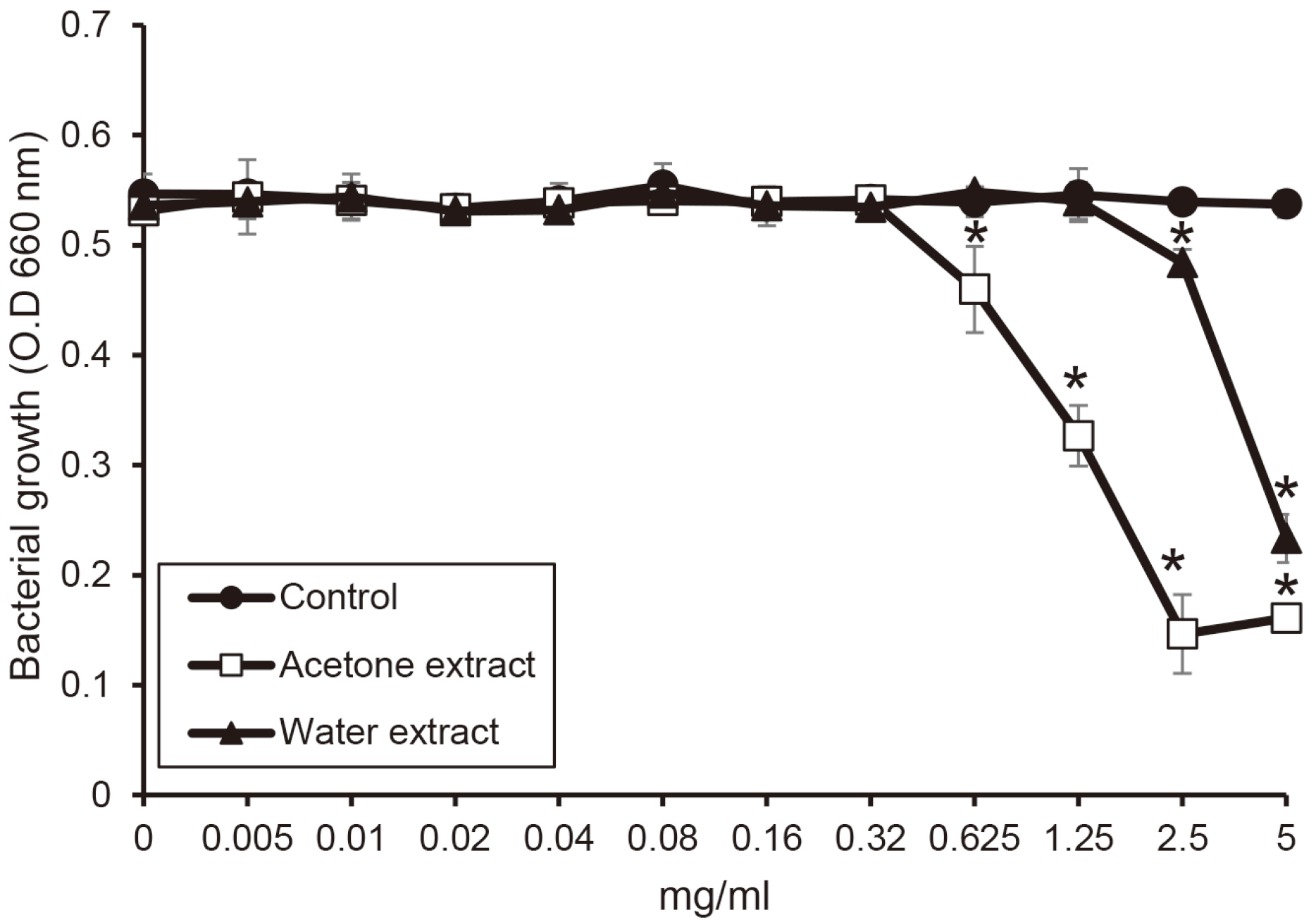
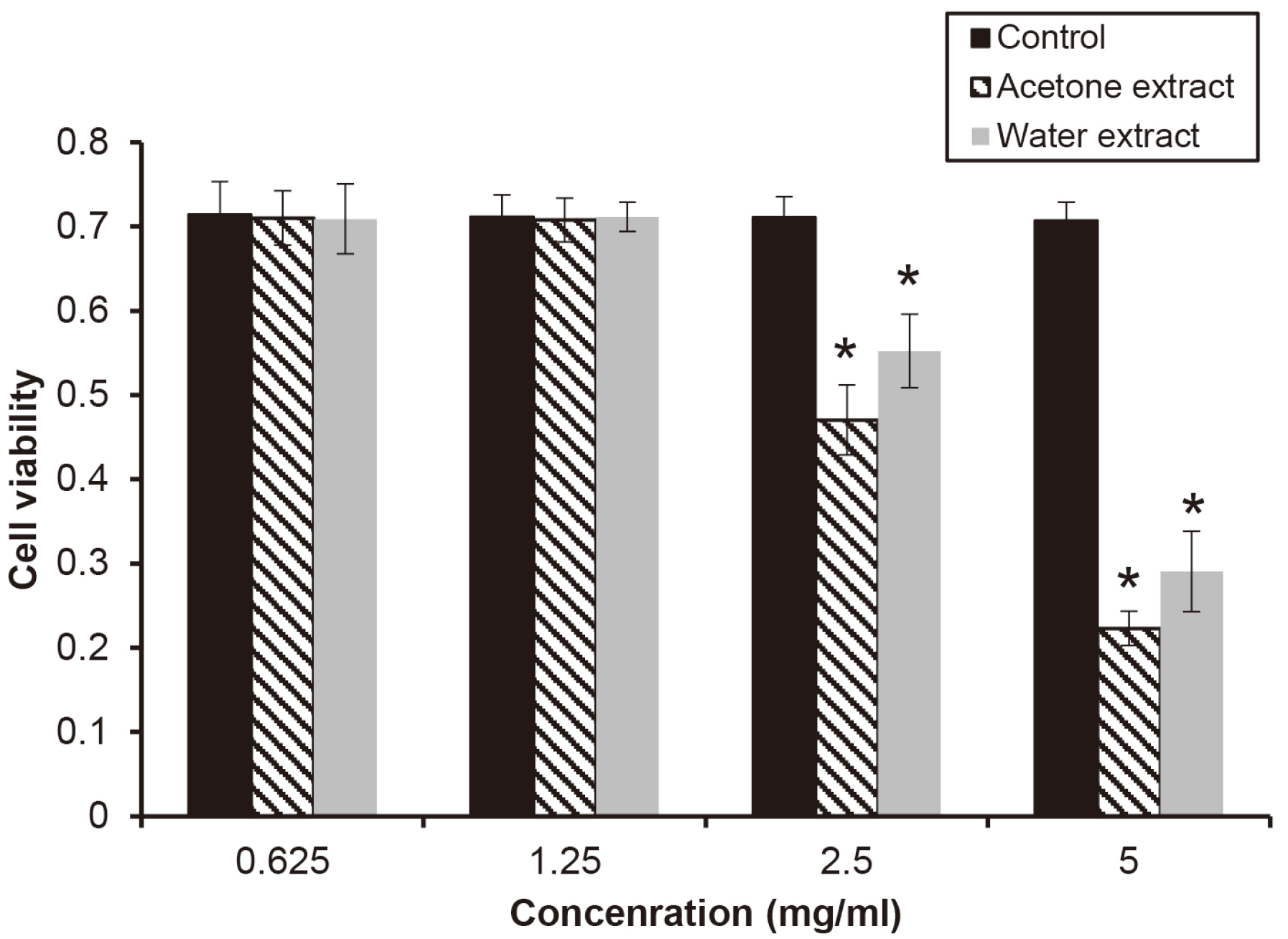
The water extract from shiitake mushroom significantly reduced the growth of periodontopathogens at 2.5 mg/ml (P < 0.05). The acetone extract significantly inhibited the growth of Porphyromonas gingivalis and Tannerella forsythia at 0.32 mg/ml and Treponema denticola growth at 0.64 mg/ml (P < 0.05). The cytotoxicity of the extract was shown at a concentration of 2.5 mg/ml. The extracts with a concentration of 1.25 mg/ml appeared to be reduce cell viability after 4 h.
Conclusion
The extracts of shiitake mushroom have antimicrobial activity against periodontitis-causing bacteria and relieving inflammation. Therefore, the extracts may be a candidate for preventing and treating periodontal disease.
Figure
Reference
-
References
1. Socransky SS, Haffajee AD. 2005; Periodontal microbial ecology. Periodontol 2000. 38:135–87. DOI: 10.1111/j.1600-0757.2005.00107.x. PMID: 15853940.
Article2. Yoo HJ, Jwa SK. 2019; Efficacy of beta-caryophyllene for periodontal disease related factors. Arch Oral Biol. 100:113–8. DOI: 10.1016/j.archoralbio.2019.02.015. PMID: 30826504.3. Gu JY, Fu ZB, Chen JL, Liu YJ, Cao XZ, Sun Y. 2022; Endotoxin tolerance induced by Porphyromonas gingivalis lipopolysaccharide alters macrophage polarization. Microb Pathog. 164:105448. DOI: 10.1016/j.micpath.2022.105448. PMID: 35189277.
Article4. Kim YJ, Lee SH. 2014; Reducing the bioactivity of Tannerella forsythia lipopolysaccharide by Porphyromonas gingivalis. J Microbiol. 52:702–8. DOI: 10.1007/s12275-014-4324-5. PMID: 25098564.
Article5. Dahle UR, Tronstad L, Olsen I. 1996; 3-hydroxy fatty acids in a lipopolysaccharide-like material from Treponema denticola strain FM. Endod Dent Traumatol. 12:202–5. DOI: 10.1111/j.1600-9657.1996.tb00515.x. PMID: 9028185.
Article6. Lee SH, Baek DH. 2013; Characteristics of Porphyromonas gingivalis lipopolysaccharide in co-culture with Fusobacterium nucleatum. Mol Oral Microbiol. 28:230–8. DOI: 10.1111/omi.12020. PMID: 23347381.
Article7. Reis FS, Barros L, Matrins A, Ferreira I. 2012; Chemical composition and nutritional value of the most widely appreciated cultivated mushrooms: an interspecies comparative study. Food Chem Toxicol. 50:191–7. DOI: 10.1016/j.fct.2011.10.056. PMID: 22056333.
Article8. Lull C, Wichers HJ, Savelkoul HF. 2005; Antiinflammatory and immunomodulating properties of fungal metabolites. Mediators Inflamm. 2005:63–80. DOI: 10.1155/MI.2005.63. PMID: 16030389. PMCID: PMC1160565.
Article9. Chien RC, Yen MT, Mau JL. 2016; Antimicrobial and antitumor activities of chitosan from shiitake stipes, compared to commercial chitosan from crab shells. Carbohydr Polym. 138:259–64. DOI: 10.1016/j.carbpol.2015.11.061. PMID: 26794761.
Article10. Wang X, Zhang L. 2009; Physicochemical properties and antitumor activities for sulfated derivatives of lentinan. Carbohydr Res. 344:2209–16. DOI: 10.1016/j.carres.2009.04.033. PMID: 19733344.
Article11. Kupcova K, Stefanova I, Plavcova Z, Hosek J, Hrouzek P, Kubec R. 2018; Antimicrobial, cytotoxic, antiinflammatory, and antioxidant activity of culinary processed shiitake medicinal mushroom (lentinus edodes, agaricomycetes) and its major sulfur sensory-active compound-lenthionine. Int J Med Mushrooms. 20:165–75. DOI: 10.1615/IntJMedMushrooms.2018025455. PMID: 29773008.
Article12. Shouji N, Takada K, Fukushima K, Hirasawa M. 2000; Anticaries effect of a component from shiitake (an edible mushroom). Caries Res. 34:94–8. DOI: 10.1159/000016559. PMID: 10601791.
Article13. Abuajah CI, Ogbonna AC, Osuji CM. 2015; Functional components and medicinal properties of food: a review. J Food Sci Technol. 52:2522–9. DOI: 10.1007/s13197-014-1396-5. PMID: 25892752. PMCID: PMC4397330.
Article14. Heleno SA, Barros L, Martins A, Queiroz MJRP, Santos-Buelga C, Ferreira ICFR. 2012; Phenolic, polysaccharidic, and lipidic fractions of mushrooms from Northeastern Portugal: Chemical compounds with antioxidant properties. J Agric Food Chem. 60:4634–40. DOI: 10.1021/jf300739m. PMID: 22515547.
Article15. Ohta K, Makinen KK, Loesche WJ. 1986; Purification and characterization of an enzyme produced by Treponema denticola capable of hydrolyzing synthetic trypsin substrates. Infect Immun. 53:213–20. DOI: 10.1128/iai.53.1.213-220.1986. PMID: 3013780. PMCID: PMC260099.
Article16. Hecht DW, Citron DM, Dzink-Fox J, Gregory WW, Jacobus NV, Jenkins SG, Rosenblatt JE, Schuetz AN, Wexler H. 2012. Methods for antimicrobial susceptibility testing of anaerobic bacteria; Approved standard-Eighth edition (M11-A8). Clinical and Laboratory Standards Institute;Wayne: p. 39. DOI: 10.1021/jf300739m.17. Socransky SS, Haffajee AD. 2002; Dental biofilms: difficult therapeutic targets. Periodontol 2000. 28:12–55. DOI: 10.1034/j.1600-0757.2002.280102.x. PMID: 12013340.
Article18. Ito T, Mori G, Oda Y, Hirano T, Sasaki H, Honma S, Furuya Y, Yajima Y. 2021; Clinical evaluation of periodontal pathogen levels by real-time polymerase chain reaction in peri-implantitis patients. Int J Implant Dent. 7:105. DOI: 10.1186/s40729-021-00385-0. PMID: 34613503. PMCID: PMC8493538. PMID: 8d8c074b712a4c2481e90ad2179a8ae2. PMID: 8d8c074b712a4c2481e90ad2179a8ae2.
Article19. Gaitán-Hernández R, Cortés N, Mata G. 2014; Improvement of yield of the edible and medicinal mushroom Lentinula edodes on wheat straw by use of supplemented spawn. Braz J Microbiol. 45:467–74. DOI: 10.1590/S1517-83822014000200013. PMID: 25242929. PMCID: PMC4166270.
Article20. Bisen PS, Baghel RK, Sanodiya BS, Thakur GS, Prasad GBKS. 2010; Lentinus edodes: a macrofungus with pharmacological activities. Curr Med Chem. 17:2419–30. DOI: 10.2174/092986710791698495. PMID: 20491636.
Article21. Soboleva AIu, Krasnopol'skaia LM, Fedorova GB, Katrukha GS. 2006; Antibiotic properties of the strains of the basidiomycete Lentinus edodes (Berk.) sing. Antibiot Khimioter. 51:3–8. PMID: 18035728.22. Solmaz G, Ozen F, Ekinci Y, Bird PS, Korachi M. 2013; Inhibitory and disruptive effects of shiitake mushroom (lentinula edodes) essential oil extract on oral biofilms. Jundishapur J Microbiol. 6:e9058. DOI: 10.5812/jjm.9058.
Article23. Burt S. 2004; Essential oils: their antibacterial properties and potential applications in foods - a review. Int J Food Microbiol. 94:223–53. DOI: 10.1016/j.ijfoodmicro.2004.03.022. PMID: 15246235.24. Yildiz MO, Celik H, Caglayan C, Genç A, Doğan T, Satici E. 2022; Neuroprotective effects of carvacrol against cadmium-induced neurotoxicity in rats: role of oxidative stress, inflammation and apoptosis. Metab Brain Dis. 37:1259–69. DOI: 10.1007/s11011-022-00945-2. PMID: 35316447.
Article25. Fan K, Li X, Cao Y, Qi H, Li L, Zhang Q, Sun H. 2015; Carvacrol inhibits proliferation and induces apoptosis in human colon cancer cells. Anticancer Drugs. 26:813–23. DOI: 10.1097/CAD.0000000000000263. PMID: 26214321.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Preventive effects of shiitake mushroom extract on candida stomatitis
- Antimicorbial effect of Zea Mays L. and Magnoliae cortex extract mixtures on periodontal pathogen and effect on human gingival fibroblast cellular activity
- Extracellular Enzyme Activities of the Monokaryotic Strains Generated from Basidiospores of Shiitake Mushroom
- Two Cases of Mushroom Intolerance
- Antimicrobial Effect of Coptidis rhizome Extract against Mutans Streptococci and Periodontopathogens