J Stroke.
2022 Sep;24(3):363-371. 10.5853/jos.2022.01004.
Cerebral Venous Reflux and Dilated Basal Ganglia Perivascular Space in Hypertensive Intracerebral Hemorrhage
- Affiliations
-
- 1Department of Neurology, National Taiwan University Hospital Bei-Hu Branch, Taipei, Taiwan
- 2Department of Neurology, National Taiwan University Hospital, Taipei, Taiwan
- 3Department of Medical Imaging, National Taiwan University Hospital, Taipei, Taiwan
- KMID: 2534265
- DOI: http://doi.org/10.5853/jos.2022.01004
Abstract
- Background and Purpose
Cerebral venous flow alterations potentially contribute to age-related white matter changes, but their role in small vessel disease has not been investigated.
Methods
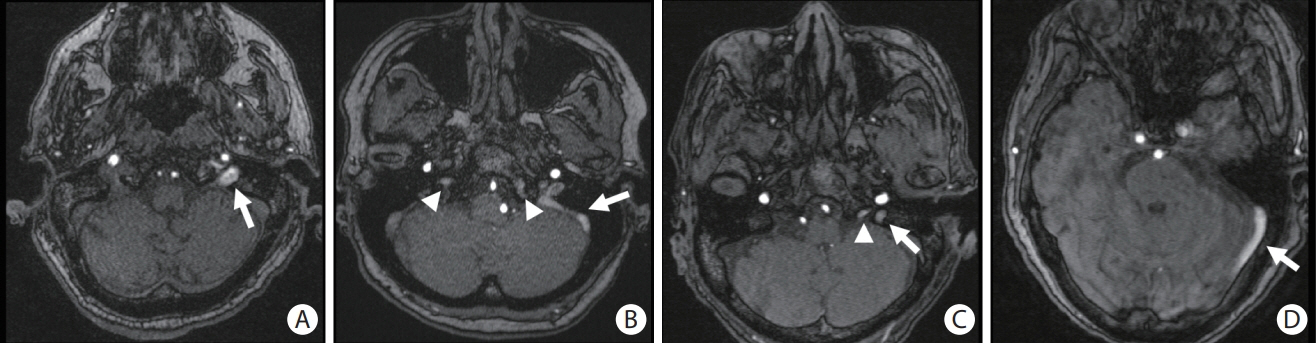
This study included 297 patients with hypertensive intracerebral hemorrhages (ICH) who underwent magnetic resonance imaging. Cerebral venous reflux (CVR) was defined as the presence of abnormal signal intensity in the dural venous sinuses or internal jugular vein on time-of-flight angiography. We investigated the association between CVR, dilated perivascular spaces (PVS), and recurrent stroke risk.
Results
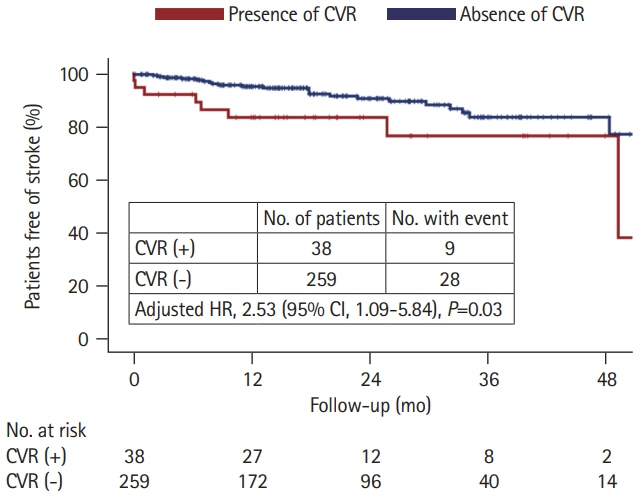
CVR was observed in 38 (12.8%) patients. Compared to patients without CVR those with CVR were more likely to have high grade (>20 in the number) dilated PVS in the basal ganglia (60.5% vs. 35.1%; adjusted odds ratio [aOR], 2.64; 95% confidence interval [CI], 1.25 to 5.60; P=0.011) and large PVS (>3 mm in diameter) (50.0% vs. 18.5%; aOR, 3.87; 95% CI, 1.85 to 8.09; P<0.001). During a median follow-up of 18 months, patients with CVR had a higher recurrent stroke rate (13.6%/year vs. 6.2%/year; aOR, 2.53; 95% CI, 1.09 to 5.84; P=0.03) than those without CVR.
Conclusions
CVR may contribute to the formation of enlarged PVS and increase the risk of recurrent stroke in patients with hypertensive ICH.
Keyword
Figure
Reference
-
References
1. Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001; 344:1450–1460.2. Tsai HH, Kim JS, Jouvent E, Gurol ME. Updates on prevention of hemorrhagic and lacunar strokes. J Stroke. 2018; 20:167–179.3. Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013; 12:822–838.4. Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol. 2019; 18:684–696.5. Fulop GA, Tarantini S, Yabluchanskiy A, Molnar A, Prodan CI, Kiss T, et al. Role of age-related alterations of the cerebral venous circulation in the pathogenesis of vascular cognitive impairment. Am J Physiol Heart Circ Physiol. 2019; 316:H1124–H1140.6. Chung CP, Beggs C, Wang PN, Bergsland N, Shepherd S, Cheng CY, et al. Jugular venous reflux and white matter abnormalities in Alzheimer’s disease: a pilot study. J Alzheimers Dis. 2014; 39:601–609.7. Chung CP, Hu HH. Pathogenesis of leukoaraiosis: role of jugular venous reflux. Med Hypotheses. 2010; 75:85–90.8. Shaaban CE, Aizenstein HJ, Jorgensen DR, MacCloud RL, Meckes NA, Erickson KI, et al. In vivo imaging of venous side cerebral small-vessel disease in older adults: an MRI method at 7T. AJNR Am J Neuroradiol. 2017; 38:1923–1928.9. Chen X, Wei L, Wang J, Shan Y, Cai W, Men X, et al. Decreased visible deep medullary veins is a novel imaging marker for cerebral small vessel disease. Neurol Sci. 2020; 41:1497–1506.10. Zhang R, Li Q, Zhou Y, Yan S, Zhang M, Lou M. The relationship between deep medullary veins score and the severity and distribution of intracranial microbleeds. Neuroimage Clin. 2019; 23:101830.11. Zhou Y, Li Q, Zhang R, Zhang W, Yan S, Xu J, et al. Role of deep medullary veins in pathogenesis of lacunes: Longitudinal observations from the CIRCLE study. J Cereb Blood Flow Metab. 2020; 40:1797–1805.12. Xu Z, Li F, Wang B, Xing D, Pei Y, Yang B, et al. New insights in addressing cerebral small vessel disease: association with the deep medullary veins. Front Aging Neurosci. 2020; 12:597799.13. Alosco ML, Brickman AM, Spitznagel MB, Griffith EY, Narkhede A, Raz N, et al. Independent and interactive effects of blood pressure and cardiac function on brain volume and white matter hyperintensities in heart failure. J Am Soc Hypertens. 2013; 7:336–343.14. Tsai HH, Chen SJ, Tsai LK, Pasi M, Lo YL, Chen YF, et al. Longterm vascular outcomes in patients with mixed location intracerebral hemorrhage and microbleeds. Neurology. 2021; 96:e995–e1004.15. Yeh SJ, Tang SC, Tsai LK, Jeng JS. Pathogenetical subtypes of recurrent intracerebral hemorrhage: designations by SMASH-U classification system. Stroke. 2014; 45:2636–2642.16. Tsai HH, Pasi M, Tsai LK, Huang CC, Chen YF, Lee BC, et al. Centrum semiovale perivascular space and amyloid deposition in spontaneous intracerebral hemorrhage. Stroke. 2021; 52:2356–2362.17. Caton MT, Callen AL, Copelan AZ, Narsinh KH, Smith ER, Amans MR. Jugular venous reflux can mimic posterior fossa dural arteriovenous fistulas on MRI-MRA. AJR Am J Roentgenol. 2021; 216:1626–1633.18. Lee J, Lee JY, Lee YJ, Park DW, Kim TY, Kim YS, et al. Differentiation of dural arteriovenous fistula from reflux venous flow on 3D TOF-MR angiography: identifying asymmetric enlargement of external carotid artery branches. Clin Radiol. 2020; 75:714.19. Kim E, Kim JH, Choi BS, Jung C, Lee DH. MRI and MR angiography findings to differentiate jugular venous reflux from cavernous dural arteriovenous fistula. AJR Am J Roentgenol. 2014; 202:839–846.20. Charidimou A, Boulouis G, Pasi M, Auriel E, van Etten ES, Haley K, et al. MRI-visible perivascular spaces in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology. 2017; 88:1157–1164.21. Doubal FN, MacLullich AM, Ferguson KJ, Dennis MS, Wardlaw JM. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke. 2010; 41:450–454.22. Ding J, Sigurðsson S, Jónsson PV, Eiriksdottir G, Charidimou A, Lopez OL, et al. Large perivascular spaces visible on magnetic resonance imaging, cerebral small vessel disease progression, and risk of dementia: the age, gene/environment susceptibility-reykjavik study. JAMA Neurol. 2017; 74:1105–1112.23. Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009; 8:165–174.24. Gregoire SM, Chaudhary UJ, Brown MM, Yousry TA, Kallis C, Jäger HR, et al. The Microbleed Anatomical Rating Scale (MARS): reliability of a tool to map brain microbleeds. Neurology. 2009; 73:1759–1766.25. Pasi M, Boulouis G, Fotiadis P, Auriel E, Charidimou A, Haley K, et al. Distribution of lacunes in cerebral amyloid angiopathy and hypertensive small vessel disease. Neurology. 2017; 88:2162–2168.26. Tsai HH, Pasi M, Tsai LK, Chen YF, Chen YW, Tang SC, et al. Superficial cerebellar microbleeds and cerebral amyloid angiopathy: a magnetic resonance imaging/positron emission tomography study. Stroke. 2020; 51:202–208.27. Tsai HH, Pasi M, Tsai LK, Chen YF, Lee BC, Tang SC, et al. Microangiopathy underlying mixed-location intracerebral hemorrhages/microbleeds: a PiB-PET study. Neurology. 2019; 92:e774–e781.28. Lichtenstein D, Saïfi R, Augarde R, Prin S, Schmitt JM, Page B, et al. The internal jugular veins are asymmetric: usefulness of ultrasound before catheterization. Intensive Care Med. 2001; 27:301–305.29. Nan D, Cheng Y, Feng L, Zhao M, Ma D, Feng J. Potential mechanism of venous system for leukoaraiosis: from postmortem to in vivo research. Neurodegener Dis. 2019; 19:101–108.30. Chung CP, Wang PN, Wu YH, Tsao YC, Sheng WY, Lin KN, et al. More severe white matter changes in the elderly with jugular venous reflux. Ann Neurol. 2011; 69:553–559.31. Wardlaw JM, Benveniste H, Nedergaard M, Zlokovic BV, Mestre H, Lee H, et al. Perivascular spaces in the brain: anatomy, physiology and pathology. Nat Rev Neurol. 2020; 16:137–153.32. Zhu YC, Tzourio C, Soumaré A, Mazoyer B, Dufouil C, Chabriat H. Severity of dilated Virchow-Robin spaces is associated with age, blood pressure, and MRI markers of small vessel disease: a population-based study. Stroke. 2010; 41:2483–2490.33. Zhang K, Zhou Y, Zhang W, Li Q, Sun J, Lou M. MRI-visible perivascular spaces in basal ganglia but not centrum semiovale or hippocampus were related to deep medullary veins changes. J Cereb Blood Flow Metab. 2022; 42:136–144.34. Zhang R, Huang P, Jiaerken Y, Wang S, Hong H, Luo X, et al. Venous disruption affects white matter integrity through increased interstitial fluid in cerebral small vessel disease. J Cereb Blood Flow Metab. 2021; 41:157–165.35. Gouveia-Freitas K, Bastos-Leite AJ. Perivascular spaces and brain waste clearance systems: relevance for neurodegenerative and cerebrovascular pathology. Neuroradiology. 2021; 63:1581–1597.36. Inano S, Itoh D, Takao H, Hayashi N, Mori H, Kunimatsu A, et al. High signal intensity in the dural sinuses on 3D-TOF MR angiography at 3.0 T. Clin Imaging. 2010; 34:332–336.37. Uchino A, Nomiyama K, Takase Y, Nakazono T, Tominaga Y, Imaizumi T, et al. Retrograde flow in the dural sinuses detected by three-dimensional time-of-flight MR angiography. Neuroradiology. 2007; 49:211–215.38. Jang J, Kim BS, Kim BY, Choi HS, Jung SL, Ahn KJ, et al. Reflux venous flow in dural sinus and internal jugular vein on 3D time-of-flight MR angiography. Neuroradiology. 2013; 55:1205–1211.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Bilateral Simultaneous Hypertensive Intracerebral Hemorrhage in Basal Ganglia
- Multiple Hypertensive Intracerebral Hemorrhage: A Case Report
- Traumatic Intracerebral Hemorrhage in Bilateral Basal Ganglia
- Multiple, Simultaneous, Hypertensive Intracerebral Hemorrhages in the Pons and Basal Ganglia: A Case Report
- Cerebrovascular Disease during Pregnancy