J Stroke.
2022 Sep;24(3):352-362. 10.5853/jos.2022.01578.
Elderly CADASIL patients with intact neurological status
- Affiliations
-
- 1Paris-Cité University, Inserm U1141 NeuroDiderot, Paris, France
- 2Department of Radiology, the Second Affiliated Hospital of Zhejiang University, School of Medicine, Hangzhou, China
- 3Department of Neurology, Amiens University Hospital, Laboratory of Functional Neurosciences1,6 (UR UPJV 4559), Jules Verne Picardy University, Amiens, France
- 4Lariboisière University Hospital, APHP, Translational Neurovascular Centre and Department of Neurology, Reference Center for Rare Vascular Diseases of the Central Nervous System and the Retina (CERVCO), FHU NeuroVasc, Paris, France
- 5Department of Geriatrics, Lariboisière University Hospital, APHP, Paris, France
- 6Department of Neurovascular Molecular Genetics, Saint-Louis Hospital, APHP, Paris, France
- KMID: 2534264
- DOI: http://doi.org/10.5853/jos.2022.01578
Abstract
- Background and Purpose
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is one of the most devastating cerebral small vessel diseases. However, despite its progression with aging, some patients remain neurologically intact (Nint) even when they get older. Their main characteristics are poorly known. We aimed to delineate their clinical, imaging, and molecular features.
Methods
Individuals aged over 65 years were selected from a cohort of 472 CADASIL patients. Subjects who had no focal deficit, cognitive impairment, or disability were considered Nint. Their demographic, genetic, clinical, and imaging features were compared to those with permanent neurological symptoms (Nps).
Results
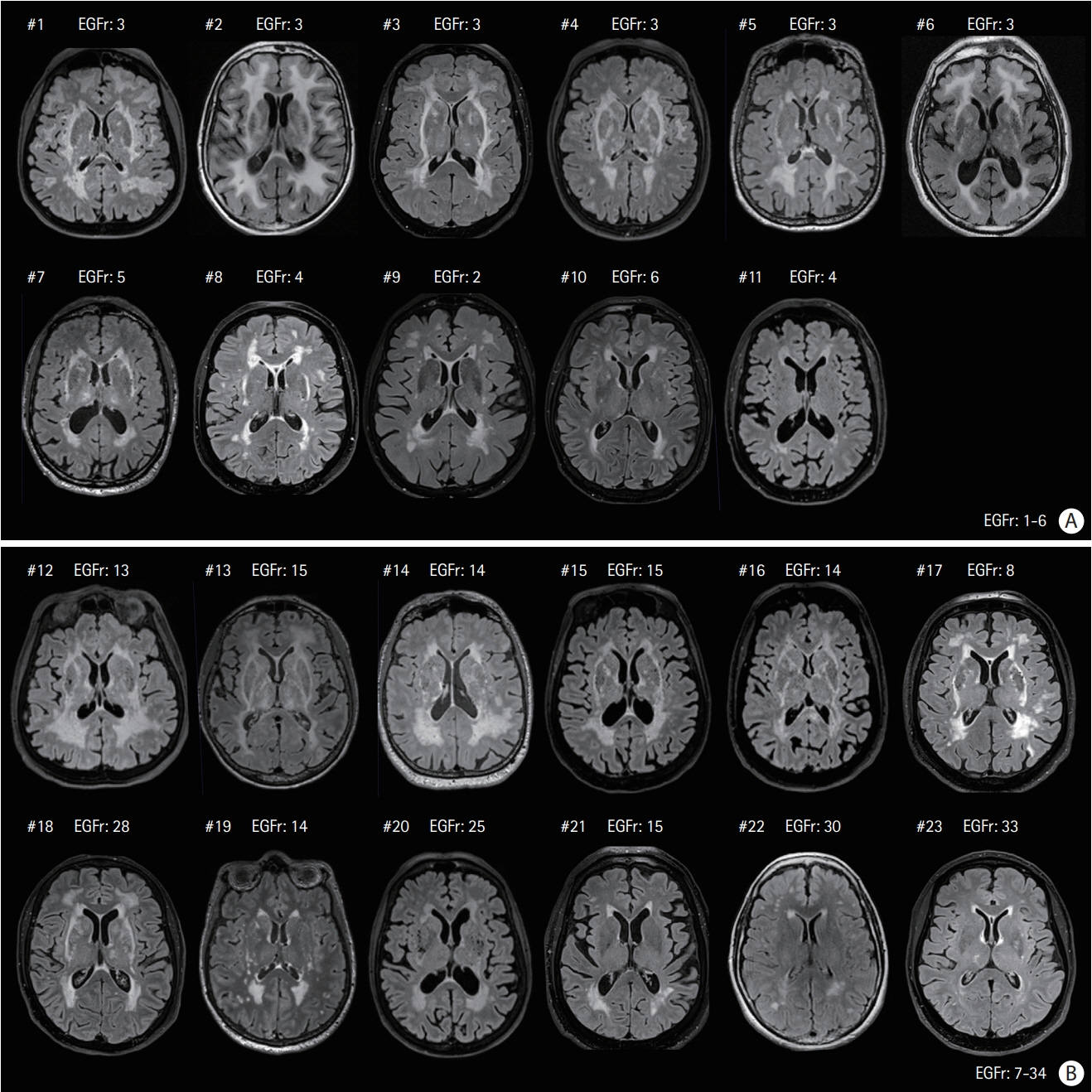
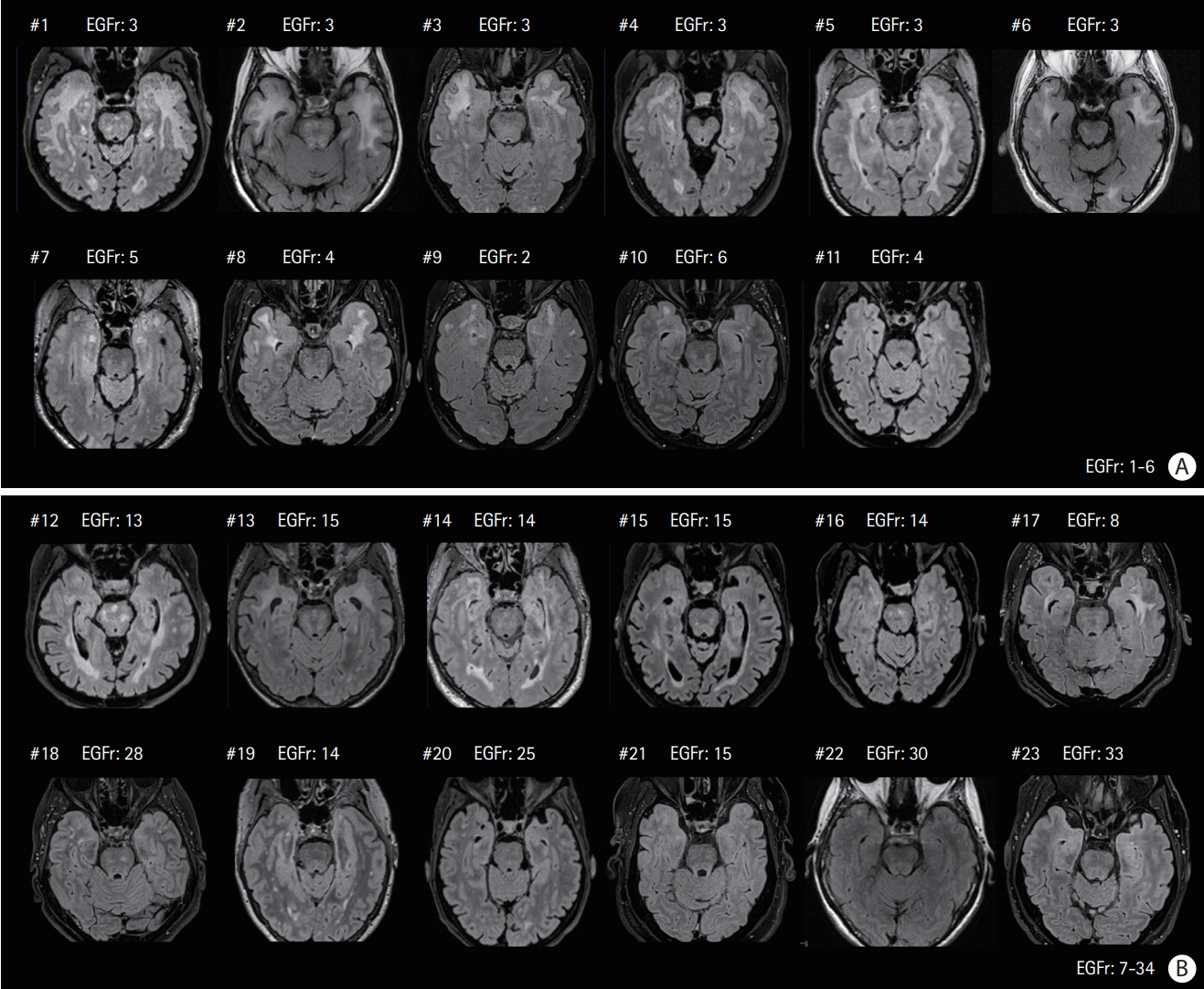
Among 129 patients, 23 (17.8%) individuals were considered Nint. The frequency of vascular risk factors and NOTCH3 cysteine mutations in epidermal growth factor-like repeat (EGFr) domains 7-34 did not differ between Nint and Nps patients but Nint patients had less stroke events and were more likely to have migraine with aura. The number of lacunes and microbleeds and degree of brain atrophy were lower in the Nint group, but the volume of white matter hyperintensities did not differ between the two groups.
Conclusions
Nearly one in five CADASIL patients can remain Nint after the age of 65 years. Their clinical and imaging profile differed from that of other age-matched CADASIL patients. The location of NOTCH3 mutation inside or outside EGFr domains 1-6 cannot fully explain this discrepancy. The factors involved in their relative preservation of brain tissue from severe damage despite aging remain to be determined.
Figure
Reference
-
References
1. Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG. Cadasil. Lancet Neurol. 2009; 8:643–653.2. Opherk C, Peters N, Herzog J, Luedtke R, Dichgans M. Longterm prognosis and causes of death in CADASIL: a retrospective study in 411 patients. Brain. 2004; 127(Pt 11):2533–2539.3. Rutten JW, Van Eijsden BJ, Duering M, Jouvent E, Opherk C, Pantoni L, et al. The effect of NOTCH3 pathogenic variant position on CADASIL disease severity: NOTCH3 EGFr 1-6 pathogenic variant are associated with a more severe phenotype and lower survival compared with EGFr 7-34 pathogenic variant. Genet Med. 2019; 21:676–682.4. Chabriat H, Vahedi K, Iba-Zizen MT, Joutel A, Nibbio A, Nagy TG, et al. Clinical spectrum of CADASIL: a study of 7 families. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Lancet. 1995; 346:934–939.5. Gesierich B, Opherk C, Rosand J, Gonik M, Malik R, Jouvent E, et al. APOE ɛ2 is associated with white matter hyperintensity volume in CADASIL. J Cereb Blood Flow Metab. 2016; 36:199–203.6. Gunda B, Hervé D, Godin O, Bruno M, Reyes S, Alili N, et al. Effects of gender on the phenotype of CADASIL. Stroke. 2012; 43:137–141.7. Adib-Samii P, Brice G, Martin RJ, Markus HS. Clinical spectrum of CADASIL and the effect of cardiovascular risk factors on phenotype: study in 200 consecutively recruited individuals. Stroke. 2010; 41:630–634.8. Ciolli L, Pescini F, Salvadori E, Del Bene A, Pracucci G, Poggesi A, et al. Influence of vascular risk factors and neuropsychological profile on functional performances in CADASIL: results from the MIcrovascular LEukoencephalopathy Study (MILES). Eur J Neurol. 2014; 21:65–71.9. Rutten JW, Dauwerse HG, Gravesteijn G, van Belzen MJ, van der Grond J, Polke JM, et al. Archetypal NOTCH3 mutations frequent in public exome: implications for CADASIL. Ann Clin Transl Neurol. 2016; 3:844–853.10. Hack RJ, Gravesteijn G, Cerfontaine MN, Hegeman IM, Mulder AA, Lesnik Oberstein SA, et al. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy family members with a pathogenic NOTCH3 variant can have a normal brain magnetic resonance imaging and skin biopsy beyond age 50 years. Stroke. 2022; 53:1964–1974.11. Gravesteijn G, Hack RJ, Opstal AM, van Eijsden BJ, Middelkoop HA, Rodriguez Girondo MD, et al. Eighteen-year disease progression and survival in CADASIL. J Stroke. 2021; 23:132–134.12. Viswanathan A, Gschwendtner A, Guichard JP, Buffon F, Cumurciuc R, O’Sullivan M, et al. Lacunar lesions are independently associated with disability and cognitive impairment in CADASIL. Neurology. 2007; 69:172–179.13. Jouvent E, Duchesnay E, Hadj-Selem F, De Guio F, Mangin JF, Hervé D, et al. Prediction of 3-year clinical course in CADASIL. Neurology. 2016; 87:1787–1795.14. Sylvestre G, Chopard G, Tio G, Magnin E, Rumbach L, Vandel P, et al. Normes diagnostiques de la batterie de tests neuropsychologiques RAPID pour les sujets âgés de 60 à 89ans présentant une maladie d’Alzheimer [Diagnostic norms of RAPID neuropsychological battery tests for subjects aged 60 to 89 years with Alzheimer’s disease]. Rev Neurol (Paris). 2011; 167:495–504.15. Jouvent E, Viswanathan A, Mangin JF, O’Sullivan M, Guichard JP, Gschwendtner A, et al. Brain atrophy is related to lacunar lesions and tissue microstructural changes in CADASIL. Stroke. 2007; 38:1786–1790.16. Ling Y, Jouvent E, Cousyn L, Chabriat H, De Guio F. Validation and optimization of BIANCA for the segmentation of extensive white matter hyperintensities. Neuroinformatics. 2018; 16:269–281.17. Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013; 12:822–838.18. Griffanti L, Zamboni G, Khan A, Li L, Bonifacio G, Sundaresan V, et al. BIANCA (Brain Intensity AbNormality Classification Algorithm): a new tool for automated segmentation of white matter hyperintensities. Neuroimage. 2016; 141:191–205.19. Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987; 149:351–356.20. Avants BB, Tustison NJ, Wu J, Cook PA, Gee JC. An open source multivariate framework for n-tissue segmentation with evaluation on public data. Neuroinformatics. 2011; 9:381–400.21. Caeiro L, Ferro JM. Cognitive profile in CADASIL patients. J Neurol Neurosurg Psychiatry. 2006; 77:144–145.22. Mourad A, Levasseur M, Bousser MG, Chabriat H. Formes pauci symptomatiques de CADASIL après 60 ans [CADASIL with minimal symptoms after 60 years]. Rev Neurol (Paris). 2006; 162:827–831. French.23. Ragno M, Fabrizi GM, Cacchiò G, Scarcella M, Sirocchi G, Selvaggio F, et al. Two novel Italian CADASIL families from Central Italy with mutation CGC-TGC at codon 1006 in the exon 19 Notch3 gene. Neurol Sci. 2006; 27:252–256.24. Pescini F, Bianchi S, Salvadori E, Poggesi A, Dotti MT, Federico A, et al. A pathogenic mutation on exon 21 of the NOTCH3 gene causing CADASIL in an octogenarian paucisymptomatic patient. J Neurol Sci. 2008; 267:170–173.25. Lee YC, Yang AH, Soong BW. The remarkably variable expressivity of CADASIL: report of a minimally symptomatic man at an advanced age. J Neurol. 2009; 256:1026–1027.26. Lewandowska E, Felczak P, Buczek J, Gramza K, Rafałowska J. Blood vessel ultrastructural picture in a CADASIL patient diagnosed at an advanced age. Folia Neuropathol. 2014; 52:443–451.27. La Piana R, Leppert IR, Pike GB, Lanthier S, Brais B, Tampieri D. 3T MRI study discloses high intrafamilial variability in CADASIL due to a novel NOTCH3 mutation. J Clin Neurosci. 2018; 58:25–29.28. Ferrante E, Mosca L, Erminio C, Penco S, Cavallari U. Identification of a novel NOTCH3 mutation in an Italian family affected by a mild form of CADASIL. Neurol Sci. 2019; 40:1751–1753.29. Gravesteijn G, Dauwerse JG, Overzier M, Brouwer G, Hegeman I, Mulder AA, et al. Naturally occurring NOTCH3 exon skipping attenuates NOTCH3 protein aggregation and disease severity in CADASIL patients. Hum Mol Genet. 2020; 29:1853–1863.30. Ragno M, Pianese L, Tiberi S, Cacchiò G, Paci C, Trojano L. First report of a homozygous mutation on exon 24 of the NOTCH3 gene in a paucisymptomatic CADASIL elderly patient. Neurol Sci. 2022; 43:1457–1458.31. Guey S, Mawet J, Hervé D, Duering M, Godin O, Jouvent E, et al. Prevalence and characteristics of migraine in CADASIL. Cephalalgia. 2016; 36:1038–1047.32. Liem MK, Oberstein SA, van der Grond J, Ferrari MD, Haan J. CADASIL and migraine: a narrative review. Cephalalgia. 2010; 30:1284–1289.33. Tan RY, Markus HS. CADASIL: migraine, encephalopathy, stroke and their inter-relationships. PLoS One. 2016; 11:e0157613.34. Chabriat H, Oberstein SL. Cognition, mood and behavior in CADASIL. Cereb Circ Cogn Behav. 2022; 3:100043.35. Wardlaw JM, Debette S, Jokinen H, De Leeuw FE, Pantoni L, Chabriat H, et al. ESO guideline on covert cerebral small vessel disease. Eur Stroke J. 2021; 6:CXI–CLXII.36. Viswanathan A, Guichard JP, Gschwendtner A, Buffon F, Cumurcuic R, Boutron C, et al. Blood pressure and haemoglobin A1c are associated with microhaemorrhage in CADASIL: a two-centre cohort study. Brain. 2006; 129(Pt 9):2375–2383.37. De Guio F, Vignaud A, Chabriat H, Jouvent E. Different types of white matter hyperintensities in CADASIL: insights from 7-Tesla MRI. J Cereb Blood Flow Metab. 2018; 38:1654–1663.38. Yao M, Jouvent E, During M, Godin O, Hervé D, Guichard JP, et al. Extensive white matter hyperintensities may increase brain volume in cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Stroke. 2012; 43:3252–3257.39. Gravesteijn G, Hack RJ, Mulder AA, Cerfontaine MN, van Doorn R, Hegeman IM, et al. NOTCH3 variant position is associated with NOTCH3 aggregation load in CADASIL vasculature. Neuropathol Appl Neurobiol. 2022; 48:e12751.40. Cho BPH, Nannoni S, Harshfield EL, Tozer D, Gräf S, Bell S, et al. NOTCH3 variants are more common than expected in the general population and associated with stroke and vascular dementia: an analysis of 200 000 participants. J Neurol Neurosurg Psychiatry. 2021; 92:694–701.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Amyotrophic Lateral Sclerosis Associated With CADASIL
- Anesthetic management of a patient with CADASIL syndrome: A case report
- CADASIL with Intracerebral Hemorrhage after Acute Ischemic Stroke
- A case of mild CADASIL patient with a novel heterozygous NOTCH3 variant
- Neuroimaging Characteristics of Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) in Korean Based on Jeju Cohort: A Pictorial Essay