Cancer Res Treat.
2022 Oct;54(4):1157-1166. 10.4143/crt.2021.997.
Aberrant DNA Methylation Maker for Predicting Metachronous Recurrence After Endoscopic Resection of Gastric Neoplasms
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- 2Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul, Korea
- 3Department of Internal Medicine, Institute of Gastroenterology, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2534195
- DOI: http://doi.org/10.4143/crt.2021.997
Abstract
- Purpose
This study aimed to investigate whether MOS methylation can be useful for the prediction of metachronous recurrence after endoscopic resection of gastric neoplasms.
Materials and Methods
From 2012 to 2017, 294 patients were prospectively enrolled after endoscopic resection of gastric dysplasia (n=171) or early gastric cancer (n=123). When Helicobacter pylori was positive, eradication therapy was performed. Among them, 124 patients completed the study protocol (follow-up duration > 3 years or development of metachronous recurrence during the follow-up). Methylation levels of MOS were measured at baseline using quantitative MethyLight assay from the antrum.
Results
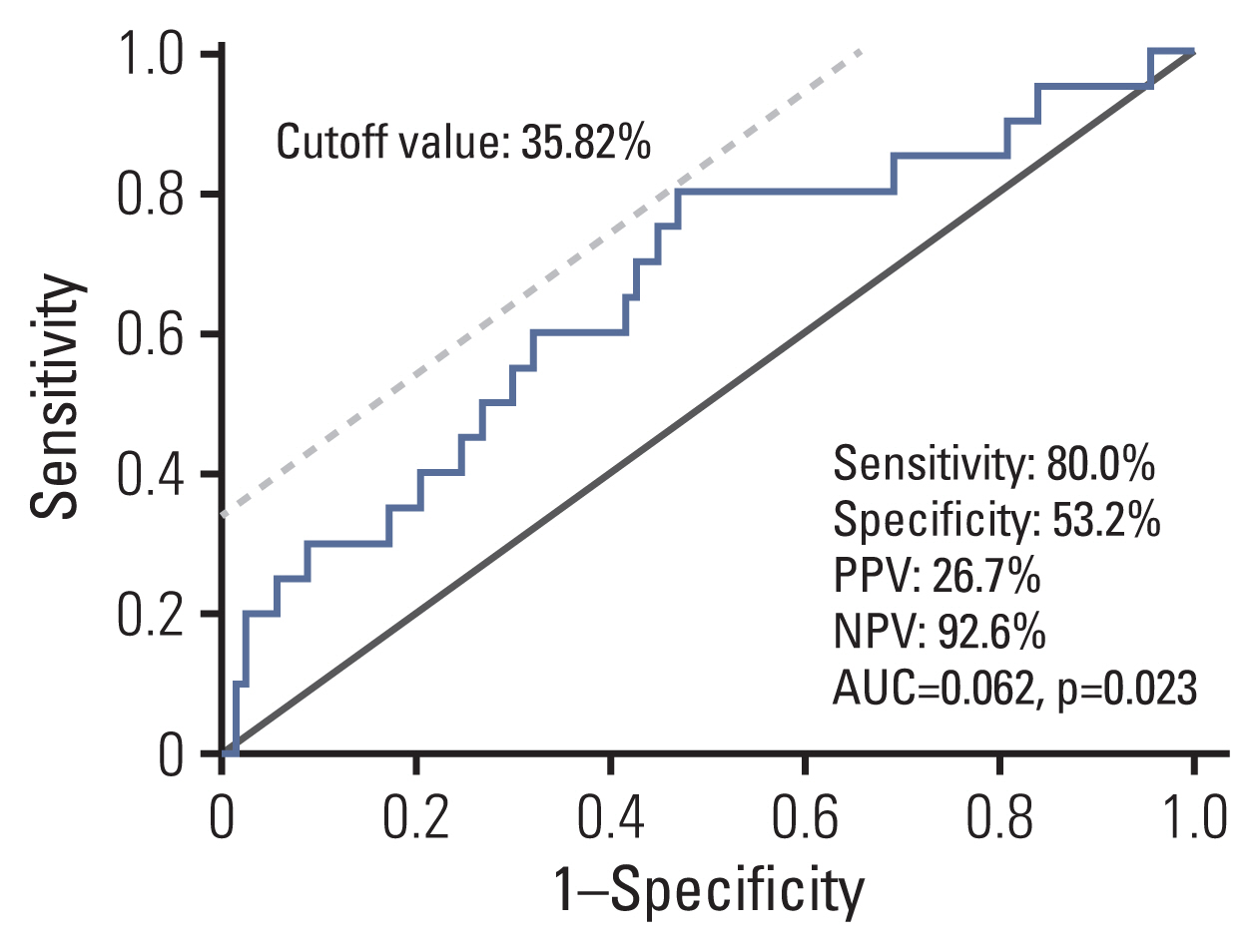
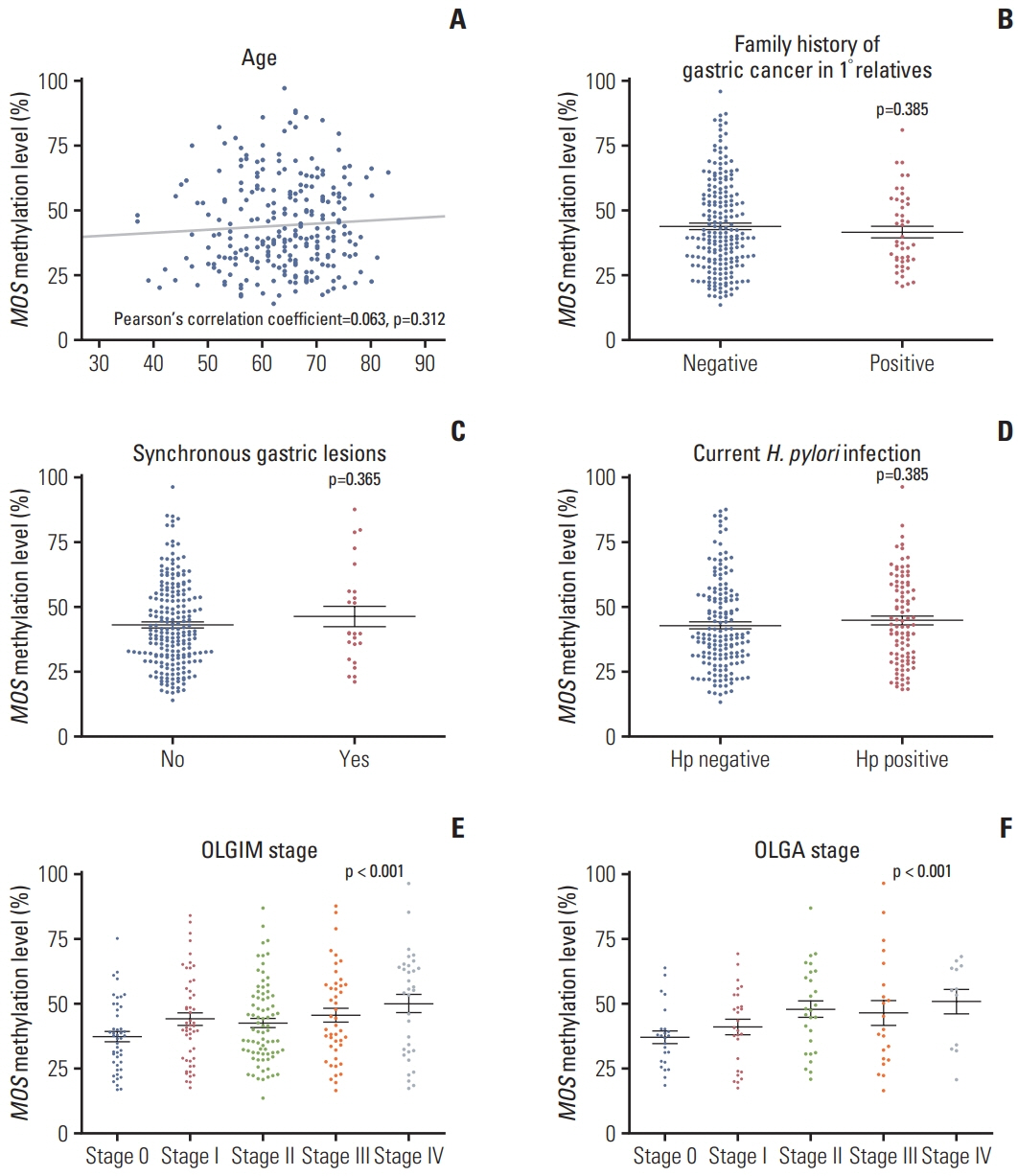
Median follow-up duration was 49.9 months. MOS methylation levels at baseline were not different by age, sex, and current H. pylorii infection, but they showed a weak correlation with operative link on gastritis assessment (OLGA) or operative link on gastric intestinal metaplasia assessment (OLGIM) stages (Spearman’s ρ=0.240 and 0.174, respectively; p < 0.05). During the follow-up, a total of 20 metachronous gastric neoplasms (13 adenomas and 7 adenocarcinomas) were developed. Either OLGA or OLGIM stage was not useful in predicting the risk for metachronous recurrence. In contrast, MOS methylation high group (≥ 34.82%) had a significantly increased risk for metachronous recurrence compared to MOS methylation low group (adjusted hazard ratio, 4.76; 95% confidence interval, 1.54 to 14.79; p=0.007).
Conclusion
MOS methylation can be a promising marker for predicting metachronous recurrence after endoscopic resection of gastric neoplasms. To confirm the usefulness of MOS methylation, validation studies are warranted in the future (ClinicalTrials No. NCT04830618).
Figure
Reference
-
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–49.2. Nakajima T, Yamashita S, Maekita T, Niwa T, Nakazawa K, Ushijima T. The presence of a methylation fingerprint of Helicobacter pylori infection in human gastric mucosae. Int J Cancer. 2009; 124:905–10.3. Shin CM, Kim N, Jung Y, Park JH, Kang GH, Kim JS, et al. Role of Helicobacter pylori infection in aberrant DNA methylation along multistep gastric carcinogenesis. Cancer Sci. 2010; 101:1337–46.4. Ushijima T. Epigenetic field for cancerization. J Biochem Mol Biol. 2007; 40:142–50.5. Padmanabhan N, Ushijima T, Tan P. How to stomach an epigenetic insult: the gastric cancer epigenome. Nat Rev Gastroenterol Hepatol. 2017; 14:467–78.6. Grady WM, Yu M, Markowitz SD. Epigenetic alterations in the gastrointestinal tract: current and emerging use for biomarkers of cancer. Gastroenterology. 2021; 160:690–709.7. Leja M, Line A. Early detection of gastric cancer beyond endoscopy: new methods. Best Pract Res Clin Gastroenterol. 2021; 50–51:101731.8. Shin CM, Kim N, Jung Y, Park JH, Kang GH, Park WY, et al. Genome-wide DNA methylation profiles in noncancerous gastric mucosae with regard to Helicobacter pylori infection and the presence of gastric cancer. Helicobacter. 2011; 16:179–88.9. Shin CM, Kim N, Park JH, Kang GH, Kim JS, Jung HC, et al. Prediction of the risk for gastric cancer using candidate methylation markers in the non-neoplastic gastric mucosae. J Pathol. 2012; 226:654–65.10. Shin CM, Kim N, Lee HS, Park JH, Ahn S, Kang GH, et al. Changes in aberrant DNA methylation after Helicobacter pylori eradication: a long-term follow-up study. Int J Cancer. 2013; 133:2034–42.11. Kim SG, Lyu DH, Park CM, Lee NR, Kim J, Cha Y, et al. Current status of endoscopic submucosal dissection for early gastric cancer in Korea: role and benefits. Korean J Intern Med. 2019; 34:785–93.12. Choi IJ, Kook MC, Kim YI, Cho SJ, Lee JY, Kim CG, et al. Helicobacter pylori therapy for the prevention of metachronous gastric cancer. N Engl J Med. 2018; 378:1085–95.13. Take S, Mizuno M, Ishiki K, Kusumoto C, Imada T, Hamada F, et al. Risk of gastric cancer in the second decade of follow-up after Helicobacter pylori eradication. J Gastroenterol. 2020; 55:281–8.14. Tan MC, Graham DY. Gastric cancer risk stratification and surveillance after Helicobacter pylori eradication: 2020. Gastrointest Endosc. 2019; 90:457–60.15. Kim N. Chemoprevention of gastric cancer by Helicobacter pylori eradication and its underlying mechanism. J Gastroenterol Hepatol. 2019; 34:1287–95.16. Hwang YJ, Choi Y, Kim N, Lee HS, Yoon H, Shin CM, et al. The difference of endoscopic and histologic improvements of atrophic gastritis and intestinal metaplasia after Helicobacter pylori eradication. Dig Dis Sci. 2022; 67:3055–66.17. Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996; 20:1161–81.18. Yun CY, Kim N, Lee J, Lee JY, Hwang YJ, Lee HS, et al. Usefulness of OLGA and OLGIM system not only for intestinal type but also for diffuse type of gastric cancer, and no interaction among the gastric cancer risk factors. Helicobacter. 2018; 23:e12542.19. Kim HJ, Kim N, Kim HW, Park JH, Shin CM, Lee DH. Promising aberrant DNA methylation marker to predict gastric cancer development in individuals with family history and long-term effects of H. pylori eradication on DNA methylation. Gastric Cancer. 2021; 24:302–13.20. Trinh BN, Long TI, Laird PW. DNA methylation analysis by MethyLight technology. Methods. 2001; 25:456–62.21. Nishizawa T, Yahagi N. Long-term outcomes of using endoscopic submucosal dissection to treat early gastric cancer. Gut Liver. 2018; 12:119–24.22. Ito M, Tanaka S, Chayama K. Characteristics and Early Dia-gnosis of gastric cancer discovered after Helicobacter pylori eradication. Gut Liver. 2021; 15:338–45.23. Suzuki R, Yamamoto E, Nojima M, Maruyama R, Yamano HO, Yoshikawa K, et al. Aberrant methylation of microRNA-34b/c is a predictive marker of metachronous gastric cancer risk. J Gastroenterol. 2014; 49:1135–44.24. Asada K, Nakajima T, Shimazu T, Yamamichi N, Maekita T, Yokoi C, et al. Demonstration of the usefulness of epigenetic cancer risk prediction by a multicentre prospective cohort study. Gut. 2015; 64:388–96.25. Maeda M, Nakajima T, Oda I, Shimazu T, Yamamichi N, Maekita T, et al. High impact of methylation accumulation on metachronous gastric cancer: 5-year follow-up of a multicentre prospective cohort study. Gut. 2017; 66:1721–3.26. Yoon H, Kim N, Shin CM, Lee HS, Kim BK, Kang GH, et al. Risk factors for metachronous gastric neoplasms in patients who underwent endoscopic resection of a gastric neoplasm. Gut Liver. 2016; 10:228–36.27. Kang HY, Kim N, Park YS, Hwang JH, Kim JW, Jeong SH, et al. Progression of atrophic gastritis and intestinal metaplasia drives Helicobacter pylori out of the gastric mucosa. Dig Dis Sci. 2006; 51:2310–5.28. Maeda M, Moro H, Ushijima T. Mechanisms for the induction of gastric cancer by Helicobacter pylori infection: aberrant DNA methylation pathway. Gastric Cancer. 2017; 20:8–15.29. Choi Y, Kim N, Yoon H, Shin CM, Park YS, Lee DH, et al. The incidence and risk factors for metachronous gastric cancer in the remnant stomach after gastric cancer surgery. Gut Liver. 2022; 16:366–74.30. Watabe H, Mitsushima T, Yamaji Y, Okamoto M, Wada R, Kokubo T, et al. Predicting the development of gastric cancer from combining Helicobacter pylori antibodies and serum pepsinogen status: a prospective endoscopic cohort study. Gut. 2005; 54:764–8.31. Terasawa T, Nishida H, Kato K, Miyashiro I, Yoshikawa T, Takaku R, et al. Prediction of gastric cancer development by serum pepsinogen test and Helicobacter pylori seropositivity in Eastern Asians: a systematic review and meta-analysis. PLoS One. 2014; 9:e109783.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- DNA Methylation as Surrogate Marker For Gastric Cancer
- Risk Factors for Metachronous Gastric Neoplasms in Patients Who Underwent Endoscopic Resection of a Gastric Neoplasm
- Helicobacter pylori Eradication and Risks of Metachronous Recurrence after Endoscopic Resection of Gastric Adenoma: A Systematic Review and Meta-Analysis
- Risk Factors for Metachronous Recurrence after Endoscopic Submucosal Dissection of a Gastric Neoplasm
- Disappearance of Serum Methylated p16 Indicates Longer Survival in Patients with Gastric Cancer