J Gastric Cancer.
2013 Sep;13(3):157-163.
Disappearance of Serum Methylated p16 Indicates Longer Survival in Patients with Gastric Cancer
- Affiliations
-
- 1Department of Surgery, Chung-Ang University College of Medicine, Seoul, Korea. jmpark@cau.ac.kr
- 2Department of Obstetrics and Gynecology, Chung-Ang University College of Medicine, Seoul, Korea.
Abstract
- PURPOSE
The aim of this study was to assess clinical correlations with postoperative alteration of p16 DNA methylation, and to clarify whether postoperative changes in the serum DNA methylation status of p16 could be used as a reliable prognostic factor for gastric cancer.
MATERIALS AND METHODS
Fifty-three consecutive gastric adenocarcinoma patients who underwent gastric resection (Chung-Ang University Hospital, Seoul, Korea) were included. DNA methylation of p16 was evaluated by methylation-specific polymerase chain reaction using serum DNA preoperatively and at the 10th postoperative day. The correlation between changes in methylation status and patients' prognosis was analyzed.
RESULTS
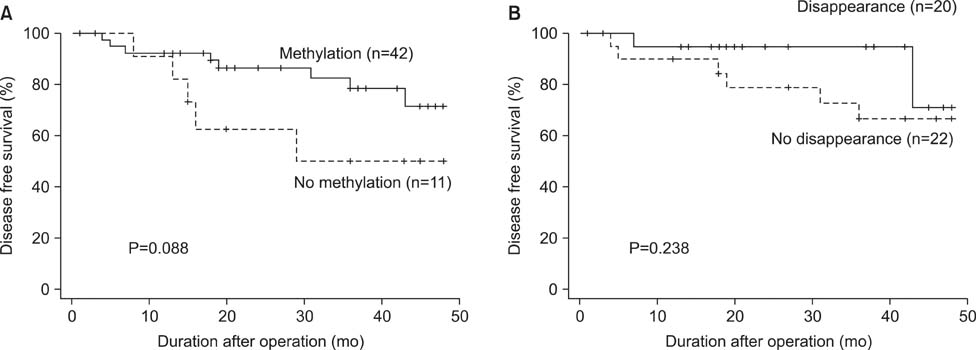
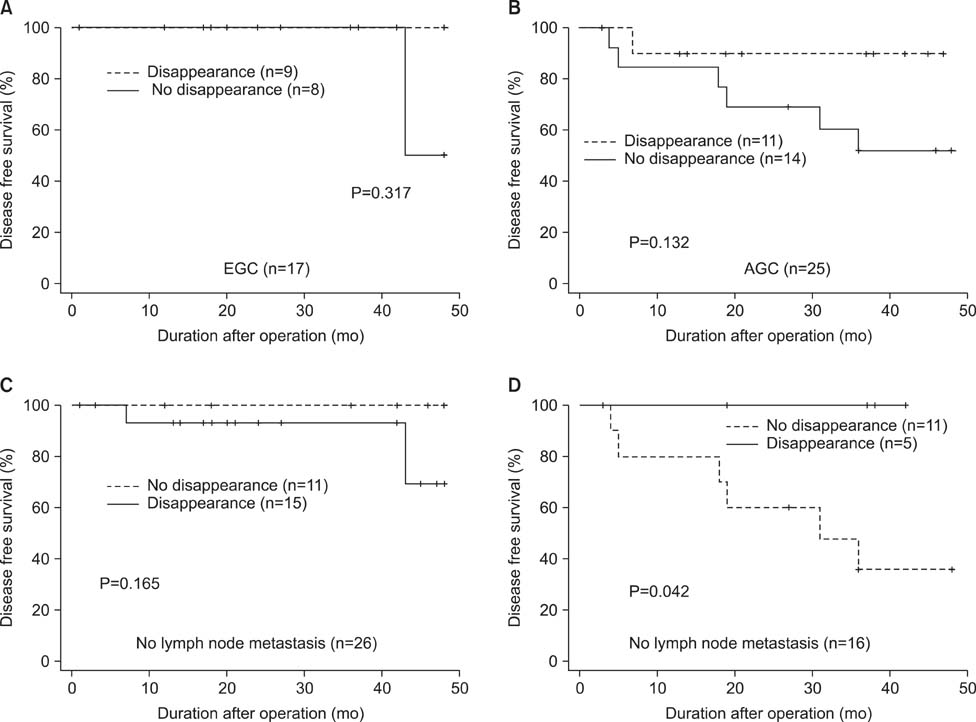
p16 was methylated in 79.2% of preoperative serum DNA and in 54.7% of postoperative serum DNA, respectively. Methylation in p16 disappeared more frequently in patients who underwent standard D2 lymphadenectomy compared to those who underwent modified D1+ lymphadenectomy (P=0.016). Whereas methylation of preoperative serum DNA was not correlated with survival, patients with postoperative disappearance of p16 methylation showed longer survival than those without postoperative disappearance of p16 methylation in the patients who had gastric cancer with lymph node metastasis (P=0.042).
CONCLUSIONS
Postoperative disappearance of p16 methylation could be an available prognostic factor for node-positive gastric cancer.
Keyword
MeSH Terms
Figure
Reference
-
1. Desai AM, Pareek M, Nightingale PG, Fielding JW. Improving outcomes in gastric cancer over 20 years. Gastric Cancer. 2004; 7:196–201.
Article2. Park JM, Ryu WS, Kim JH, Park SS, Kim SJ, Kim CS, et al. Prognostic factors for advanced gastric cancer: stage-stratified analysis of patients who underwent curative resection. Cancer Res Treat. 2006; 38:13–18.
Article3. Siewert JR, Böttcher K, Stein HJ, Roder JD. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg. 1998; 228:449–461.4. Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004; 429:457–463.
Article5. Ikoma H, Ichikawa D, Daito I, Nobuyuki T, Koike H, Okamoto K, et al. Clinical application of methylation specific-polymerase chain reaction in serum of patients with gastric cancer. Hepatogastroenterology. 2007; 54:946–950.6. Kang GH, Shim YH, Jung HY, Kim WH, Ro JY, Rhyu MG. CpG island methylation in premalignant stages of gastric carcinoma. Cancer Res. 2001; 61:2847–2851.7. Toyota M, Ahuja N, Suzuki H, Itoh F, Ohe-Toyota M, Imai K, et al. Aberrant methylation in gastric cancer associated with the CpG island methylator phenotype. Cancer Res. 1999; 59:5438–5442.8. Oue N, Sentani K, Yokozaki H, Kitadai Y, Ito R, Yasui W. Promoter methylation status of the DNA repair genes hMLH1 and MGMT in gastric carcinoma and metaplastic mucosa. Pathobiology. 2001; 69:143–149.
Article9. Lee JH, Park SJ, Abraham SC, Seo JS, Nam JH, Choi C, et al. Frequent CpG island methylation in precursor lesions and early gastric adenocarcinomas. Oncogene. 2004; 23:4646–4654.
Article10. Kang GH, Lee S, Kim JS, Jung HY. Profile of aberrant CpG island methylation along the multistep pathway of gastric carcinogenesis. Lab Invest. 2003; 83:635–641.
Article11. Oue N, Mitani Y, Motoshita J, Matsumura S, Yoshida K, Kuniyasu H, et al. Accumulation of DNA methylation is associated with tumor stage in gastric cancer. Cancer. 2006; 106:1250–1259.
Article12. Goto T, Mizukami H, Shirahata A, Yokomizo K, Kitamura YH, Sakuraba K, et al. Methylation of the p16 gene is frequently detected in lymphatic-invasive gastric cancer. Anticancer Res. 2010; 30:2701–2703.13. Mitsuno M, Kitajima Y, Ide T, Ohtaka K, Tanaka M, Satoh S, et al. Aberrant methylation of p16 predicts candidates for 5-fluorouracil-based adjuvant therapy in gastric cancer patients. J Gastroenterol. 2007; 42:866–873.
Article14. Sobin LH, Gospodarowicz MK, Wittekind C. International Union against Cancer. TNM Classification of Malignant Tumours. 7th ed. West Sussex, Hoboken: Blackwell Publisging Ltd.;2010.15. Herman JG, Graff JR, Myöhänen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci U S A. 1996; 93:9821–9826.
Article16. An C, Choi IS, Yao JC, Worah S, Xie K, Mansfield PF, et al. Prognostic significance of CpG island methylator phenotype and microsatellite instability in gastric carcinoma. Clin Cancer Res. 2005; 11:656–663.17. Shi J, Zhang G, Yao D, Liu W, Wang N, Ji M, et al. Prognostic significance of aberrant gene methylation in gastric cancer. Am J Cancer Res. 2012; 2:116–129.18. Tsujie M, Yamamoto H, Tomita N, Sugita Y, Ohue M, Sakita I, et al. Expression of tumor suppressor gene p16(INK4) products in primary gastric cancer. Oncology. 2000; 58:126–136.
Article19. Hibi K, Robinson CR, Booker S, Wu L, Hamilton SR, Sidransky D, et al. Molecular detection of genetic alterations in the serum of colorectal cancer patients. Cancer Res. 1998; 58:1405–1407.20. Esteller M, Sanchez-Cespedes M, Rosell R, Sidransky D, Baylin SB, Herman JG. Detection of aberrant promoter hypermethylation of tumor suppressor genes in serum DNA from nonsmall cell lung cancer patients. Cancer Res. 1999; 59:67–70.21. Wong IH, Lo YM, Zhang J, Liew CT, Ng MH, Wong N, et al. Detection of aberrant p16 methylation in the plasma and serum of liver cancer patients. Cancer Res. 1999; 59:71–73.22. Lee TL, Leung WK, Chan MW, Ng EK, Tong JH, Lo KW, et al. Detection of gene promoter hypermethylation in the tumor and serum of patients with gastric carcinoma. Clin Cancer Res. 2002; 8:1761–1766.23. Leung WK, To KF, Chu ES, Chan MW, Bai AH, Ng EK, et al. Potential diagnostic and prognostic values of detecting promoter hypermethylation in the serum of patients with gastric cancer. Br J Cancer. 2005; 92:2190–2194.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Methylation of the p16 tumor suppressor gene in Korean patients with colon cancer and adenoma
- The p16INK4A Expression in Stomach Cancer , Colon Cancer and Hepatoma Cell Lines
- DNA Methylation in Lung Cancer
- Methylation Patterns of Cancer-Associated Genes in Breast Cancer
- Expression of p14(ARF), p16(INK4a), p53, pRb in Gastric Cancer