Ann Pediatr Endocrinol Metab.
2022 Sep;27(3):236-241. 10.6065/apem.2142044.022.
Low-dose mitotane-induced neurological and endocrinological complication in a 5-year-old girl with adrenocortical carcinoma
- Affiliations
-
- 1Department of Pediatrics, Seoul National University Children’s Hospital, Seoul National University College of Medicine, Seoul, Korea
- 2Department of Pediatrics, Dong-A University Hospital, Dong-A University College of Medicine, Busan, Korea
- KMID: 2533344
- DOI: http://doi.org/10.6065/apem.2142044.022
Abstract
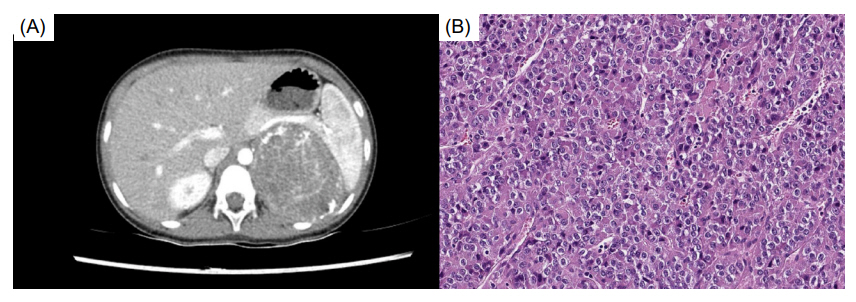
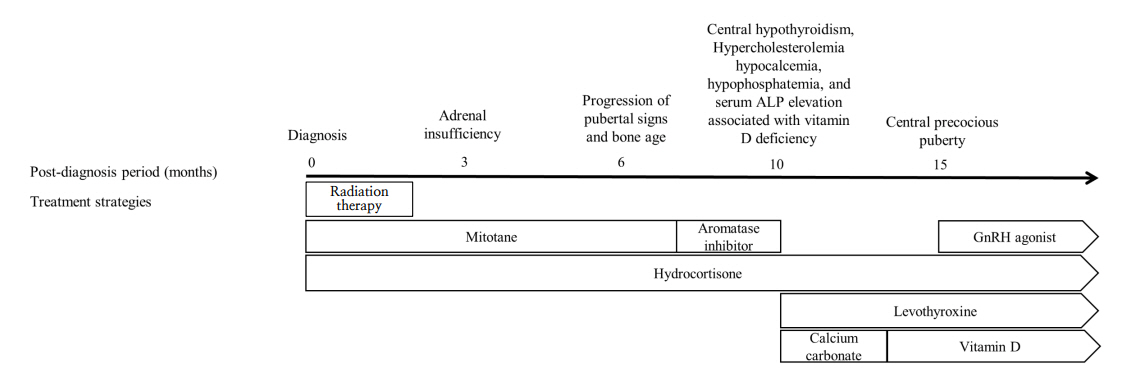
- Mitotane is an adrenolytic drug that exhibits therapeutic effects within a narrow target range (14–20 μg/dL). Various complications develop if the upper limit is exceeded. We present the case of a 5-year-old girl with breast development, acne, and pubic hair who was diagnosed with an adrenal mass that was subsequently excised. The pathological finding was adrenocortical carcinoma with a high risk of malignancy, and adjuvant therapy (combined mitotane and radiation therapy) was recommended. Mitotane was initiated at a low dose to allow monitoring of the therapeutic drug level, and high-dose hydrocortisone was also administered. However, the patient exhibited elevated adrenocorticotropic hormone levels and vague symptoms such as general weakness and difficulty concentrating. It was important to determine if these symptoms were signs of the neurological complications that develop when mitotane level is elevated. Encephalopathy progression and pubertal signs appeared 6 months after diagnosis, induced by high mitotane level. The mitotane decreased to subtherapeutic level several months after its discontinuation, at which time endocrinopathy (central hypothyroidism, hypercholesterolemia, and secondary central precocious puberty) developed. The case shows that low-dose mitotane can trigger neurological and endocrinological complications in a pediatric patient, indicating that the drug dose should be individualized with frequent monitoring of the therapeutic level.
Figure
Cited by 1 articles
-
Overview of endocrine tumor syndromes manifesting as adrenal tumors
Ja Hye Kim
Ewha Med J. 2024;47(1):e4. doi: 10.12771/emj.2024.e4.
Reference
-
References
1. Schteingart DE, Doherty GM, Gauger PG, Giordano TJ, Hammer GD, Korobkin M, et ar. Management of patients with adrenal cancer: recommendations of an international consensus conference. Endocr Relat Cancer. 2005; 12:667–80.2. Fassnacht M, Dekkers OM, Else T, Baudin E, Berruti A, de Krijger R, et al. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2018; 179:G1–46.3. Tang Y, Liu Z, Zou Z, Liang J, Lu Y, Zhu Y. Benefits of adjuvant mitotane after resection of adrenocortical carcinoma: a systematic review and meta-analysis. Biomed Res Int. 2018; 2018:9362108.4. Baudin E, Pellegriti G, Bonnay M, Penfornis A, Laplanche A, Vassal G, et al. Impact of monitoring plasma 1,1-dichloro diphenildichloroethane (o,p'DDD) levels on the treatment of patients with adrenocortical carcinoma. Cancer. 2001; 92:1385–92.5. Terzolo M, Pia A, Berruti A, Osella G, Alì A, Carbone V, et al. Low-dose monitored mitotane treatment achieves the therapeutic range with manageable side effects in patients with adrenocortical cancer. J Clin Endocrinol Metab. 2000; 85:2234–8.6. Faggiano A, Leboulleux S, Young J, Schlumberger M, Baudin E. Rapidly progressing high o,p'DDD doses shorten the time required to reach the therapeutic threshold with an acceptable tolerance: preliminary results. Clin Endocrinol (Oxf). 2006; 64:110–3.7. Michalkiewicz E, Sandrini R, Figueiredo B, Miranda EC, Caran E, Oliveira-Filho AG, et al. Clinical and outcome characteristics of children with adrenocortical tumors: a report from the International Pediatric Adrenocortical Tumor Registry. J Clin Oncol. 2004; 22:838–45.8. Zancanella P, Pianovski MA, Oliveira BH, Ferman S, Piovezan GC, Lichtvan LL, et al. Mitotane associated with cisplatin, etoposide, and doxorubicin in advanced childhood adrenocortical carcinoma: mitotane monitoring and tumor regression. J Pediatr Hematol Oncol. 2006; 28:513–24.9. Kerkhofs TM, Ettaieb MH, Verhoeven RH, Kaspers GJ, Tissing WJ, Loeffen J, et al. Adrenocortical carcinoma in children: first population-based clinicopathological study with long-term follow-up. Oncol Rep. 2014; 32:2836–44.10. Goto T, Miyako K, Kuromaru R, Ihara K, Torisu H, Sanefuji M, et al. Case report: adjuvant therapy with a high dose of mitotane for adrenocortical carcinoma in a 4-year-old boy. Clin Pediatr Endocrinol. 2008; 17:71–4.11. Else T, Kim AC, Sabolch A, Raymond VM, Kandathil A, Caoili EM, et al. Adrenocortical carcinoma. Endocr Rev. 2014; 35:282–326.12. Lindhe O, Skogseid B, Brandt I. Cytochrome P450-catalyzed binding of 3-methylsulfonyl-DDE and o,p'- DDD in human adrenal zona fasciculata/reticularis. J Clin Endocrinol Metab. 2002; 87:1319–26.13. Hescot S, Slama A, Lombès A, Paci A, Remy H, Leboulleux S, et al. Mitotane alters mitochondrial respiratory chain activity by inducing cytochrome c oxidase defect in human adrenocortical cells. Endocr Relat Cancer. 2013; 20:371–81.14. Lehmann TP, Wrzesiński T, Jagodziński PP. The effect of mitotane on viability, steroidogenesis and gene expression in NCI H295R adrenocortical cells. Mol Med Rep. 2013; 7:893–900.15. MOY RH. Studies of the pharmacology of o,p'DDD in man. J Lab Clin Med. 1961; 58:296–304.16. Lysodren 500 mg tablets [Internet]. France: HRA Pharma Rare Diseases; 2021 [Updated 2020 Oct 7; cited 2021 April 6]. Available from: http://www.medicines.org.uk/emc/product/80.17. Daffara F, De Francia S, Reimondo G, Zaggia B, Aroasio E, Porpiglia F, et al. Prospective evaluation of mitotane toxicity in adrenocortical cancer patients treated adjuvantly. Endocr Relat Cancer. 2008; 15:1043–53.18. Zatelli MC, Gentilin E, Daffara F, Tagliati F, Reimondo G, Carandina G, et al. Therapeutic concentrations of mitotane (o,p'-DDD) inhibit thyrotroph cell viability and TSH expression and secretion in a mouse cell line model. Endocrinology. 2010; 151:2453–61.19. Latronico AC, Brito VN, Carel JC. Causes, diagnosis, and treatment of central precocious puberty. Lancet Diabetes Endocrinol. 2016; 4:265–74.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Estrogen Producing Adrenocortical Carcinoma
- A Case of Adrenocortical Carcinoma Secreting Cortisol, Androgen and Aldosterone
- A Case of Cushing's Syndrome caused by Adrenocortical Carcinoma
- A Case of Adrenal Cortical Carcinoma with Invasion of Inferior Vena Cava
- A Case of an Adrenocortical Carcinoma with Pulmonary Embolism as the Initial Manifestation