Clin Endosc.
2022 Sep;55(5):665-673. 10.5946/ce.2021.265.
Preventive effect of tacrolimus on patients with post-endoscopic retrograde cholangiopancreatography pancreatitis
- Affiliations
-
- 1Department of Gastroenterology, Amrita Institute of Medical Sciences, Kochi, India
- KMID: 2533306
- DOI: http://doi.org/10.5946/ce.2021.265
Abstract
- Background/Aims
In patients undergoing endoscopic retrograde cholangiopancreatography (ERCP), calcineurin activates zymogen, which results in pancreatitis. In this study, we aimed to determine the efficacy of tacrolimus, a calcineurin inhibitor, in preventing post-ERCP pancreatitis (PEP).
Methods
This was a prospective pilot study in which patients who underwent ERCP received tacrolimus (4 mg in two divided doses); this was the Tac group. A contemporaneous cohort of patients was included as a control group. All patients were followed-up for PEP. PEP was characterized by worsening abdominal pain with an acute onset, elevated pancreatic enzymes, and a duration of hospital stay of more than 48 hours. Serum tacrolimus levels were measured immediately before the procedure in the Tac group.
Results
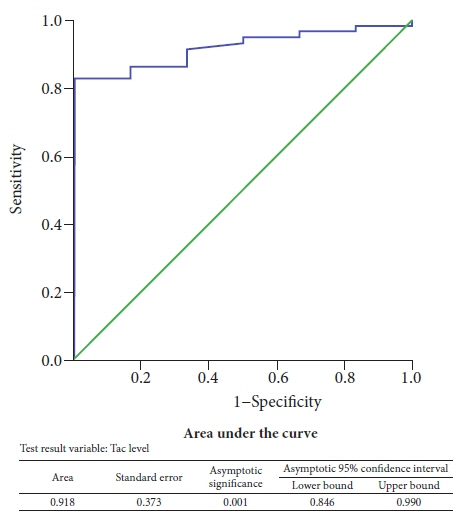
There were no differences in the baseline characteristics between the Tac group (n=48) and the control group (n=51). Only four out of 48 patients (8.3%) had PEP in the Tac group compared to eight out of 51 patients (15.7%) who had PEP in the control group. The mean trough tacrolimus level in patients who developed PEP was significantly lower (p<0.05).
Conclusions
Oral tacrolimus at a cumulative dose of 4 mg safely prevents PEP. Further randomized controlled studies are warranted to establish the role of tacrolimus in this context.
Keyword
Figure
Reference
-
1. Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010; 71:446–454.2. Gottlieb K, Sherman S. ERCP and biliary endoscopic sphincterotomy-induced pancreatitis. Gastrointest Endosc Clin N Am. 1998; 8:87–114.3. Freeman ML. Adverse outcomes of ERCP. Gastrointest Endosc. 2002; 56:S273–S282.4. Testoni PA, Mariani A, Giussani A, et al. Risk factors for post-ERCP pancreatitis in high- and low-volume centers and among expert and non-expert operators: a prospective multicenter study. Am J Gastroenterol. 2010; 105:1753–1761.5. Freeman ML, DiSario JA, Nelson DB, et al. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc. 2001; 54:425–434.6. Loperfido S, Angelini G, Benedetti G, et al. Major early complications from diagnostic and therapeutic ERCP: a prospective multicenter study. Gastrointest Endosc. 1998; 48:1–10.7. Masci E, Toti G, Mariani A, et al. Complications of diagnostic and therapeutic ERCP: a prospective multicenter study. Am J Gastroenterol. 2001; 96:417–423.8. Freeman ML, Nelson DB, Sherman S, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996; 335:909–918.9. Vandervoort J, Soetikno RM, Tham TC, et al. Risk factors for complications after performance of ERCP. Gastrointest Endosc. 2002; 56:652–656.10. Cheng CL, Sherman S, Watkins JL, et al. Risk factors for post-ERCP pancreatitis: a prospective multicenter study. Am J Gastroenterol. 2006; 101:139–147.11. Cotton PB, Garrow DA, Gallagher J, et al. Risk factors for complications after ERCP: a multivariate analysis of 11,497 procedures over 12 years. Gastrointest Endosc. 2009; 70:80–88.12. Wang P, Li ZS, Liu F, et al. Risk factors for ERCP-related complications: a prospective multicenter study. Am J Gastroenterol. 2009; 104:31–40.13. Pezzilli R, Romboli E, Campana D, et al. Mechanisms involved in the onset of post-ERCP pancreatitis. JOP. 2002; 3:162–168.14. Wang ZK, Yang YS, Cai FC, et al. Is prophylactic somatostatin effective to prevent post-endoscopic retrograde cholangiopancreatography pancreatitis or hyperamylasemia? A randomized, placebo-controlled pilot trial. Chin Med J (Engl). 2013; 126:2403–2408.15. Andriulli A, Clemente R, Solmi L, et al. Gabexate or somatostatin administration before ERCP in patients at high risk for post-ERCP pancreatitis: a multicenter, placebo-controlled, randomized clinical trial. Gastrointest Endosc. 2002; 56:488–495.16. Zhang Y, Chen QB, Gao ZY, et al. Meta-analysis: octreotide prevents post-ERCP pancreatitis, but only at sufficient doses. Aliment Pharmacol Ther. 2009; 29:1155–1164.17. Yaghoobi M, Rolland S, Waschke KA, et al. Meta-analysis: rectal indomethacin for the prevention of post-ERCP pancreatitis. Aliment Pharmacol Ther. 2013; 38:995–1001.18. Sun HL, Han B, Zhai HP, et al. Rectal NSAIDs for the prevention of post-ERCP pancreatitis: a meta-analysis of randomized controlled trials. Surgeon. 2014; 12:141–147.19. Yuhara H, Ogawa M, Kawaguchi Y, et al. Pharmacologic prophylaxis of post-endoscopic retrograde cholangiopancreatography pancreatitis: protease inhibitors and NSAIDs in a meta-analysis. J Gastroenterol. 2014; 49:388–399.20. Sethi S, Sethi N, Wadhwa V, et al. A meta-analysis on the role of rectal diclofenac and indomethacin in the prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis. Pancreas. 2014; 43:190–197.21. Elmunzer BJ, Higgins PD, Saini SD, et al. Does rectal indomethacin eliminate the need for prophylactic pancreatic stent placement in patients undergoing high-risk ERCP? Post hoc efficacy and cost-benefit analyses using prospective clinical trial data. Am J Gastroenterol. 2013; 108:410–415.22. Tse F, Yuan Y, Moayyedi P, et al. Guide wire-assisted cannulation for the prevention of post-ERCP pancreatitis: a systematic review and meta-analysis. Endoscopy. 2013; 45:605–618.23. Andriulli A, Forlano R, Napolitano G, et al. Pancreatic duct stents in the prophylaxis of pancreatic damage after endoscopic retrograde cholangiopancreatography: a systematic analysis of benefits and associated risks. Digestion. 2007; 75:156–163.24. Mazaki T, Mado K, Masuda H, et al. Prophylactic pancreatic stent placement and post-ERCP pancreatitis: an updated meta-analysis. J Gastroenterol. 2014; 49:343–355.25. Orabi AI, Wen L, Javed TA, et al. Targeted inhibition of pancreatic acinar cell calcineurin is a novel strategy to prevent post-ERCP pancreatitis. Cell Mol Gastroenterol Hepatol. 2017; 3:119–128.26. Thiruvengadam NR, Forde KA, Chandrasekhara V, et al. Tacrolimus and indomethacin are safe and effective at reducing pancreatitis after endoscopic retrograde cholangiopancreatography in patients who have undergone liver transplantation. Clin Gastroenterol Hepatol. 2020; 18:1224–1232.27. Holt DW. Therapeutic drug monitoring of immunosuppressive drugs in kidney transplantation. Curr Opin Nephrol Hypertens. 2002; 11:657–663.28. ASGE Standards of Practice Committee, Chandrasekhara V, Khashab MA, et al. Adverse events associated with ERCP. Gastrointest Endosc. 2017; 85:32–47.29. Law R, Leal C, Dayyeh BA, et al. Role of immunosuppression in post-endoscopic retrograde cholangiopancreatography pancreatitis after liver transplantation: a retrospective analysis. Liver Transpl. 2013; 19:1354–1360.30. Bai Y, Gao J, Shi X, et al. Prophylactic corticosteroids do not prevent post-ERCP pancreatitis: a meta-analysis of randomized controlled trials. Pancreatology. 2008; 8:504–509.31. Yang Z, Peng Y, Wang S. Immunosuppressants: pharmacokinetics, methods of monitoring and role of high performance liquid chromatography/mass spectrometry. Clin Appl Immunol Rev. 2005; 5:405–430.32. Baillie J. Fifteen years of ERCP. Gastrointest Endosc. 2017; 86:327–328.33. Xu J, Xu L, Wei X, et al. A case report: acute pancreatitis associated with tacrolimus in kidney transplantation. BMC Nephrol. 2019; 20:209.34. Nieto Y, Russ P, Everson G, et al. Acute pancreatitis during immunosuppression with tacrolimus following an allogeneic umbilical cord blood transplantation. Bone Marrow Transplant. 2000; 26:109–111.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prevention of Post-endoscopic Retrograde Cholangiopancreatography Pancreatitis: An Endoscopic Perspective
- Prevention of Postendoscopic Retrograde Cholangiopancreatography Pancreatitis: The Endoscopic Technique
- Endoscopic retrograde cholangiopancreatography complications: Techniques to reduce risk and management strategies
- Tacrolimus for prophylaxis of post-endoscopic retrograde cholangiopancreatography pancreatitis: a potential new target of old drug?
- Prevention and Management of Post-Endoscopic Retrograde Cholangiopancreatography Complications