Clin Endosc.
2022 Sep;55(5):637-644. 10.5946/ce.2021.257.
Comparison of diagnostic performances of slow-pull suction and standard suction in endoscopic ultrasound-guided fine needle biopsy for gastrointestinal subepithelial tumors
- Affiliations
-
- 1Department of Internal Medicine, Kyungpook National University School of Medicine, Daegu, Korea
- 2Department of Pathology, Kyungpook National University School of Medicine, Daegu, Korea
- KMID: 2533303
- DOI: http://doi.org/10.5946/ce.2021.257
Abstract
- Background/Aims
Endoscopic ultrasound-guided fine-needle biopsy (EUS-FNB) is integral to the diagnosis of gastrointestinal (GI) subepithelial tumors (SETs). The impact of different EUS-FNB tissue sampling techniques on specimen adequacy and diagnostic accuracy in SETs has not been fully evaluated. This study aimed to compare the diagnostic outcomes of slow-pull (SP) and standard suction (SS) in patients with GI SETs.
Methods
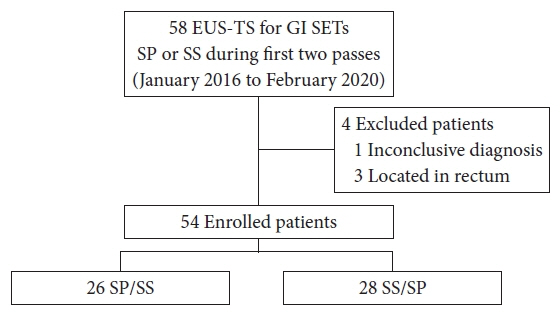
In this retrospective comparative study, 54 patients were enrolled. Medical records were reviewed for location and size of the target lesion, FNB needle type/size, technical order, specimen adequacy, diagnostic yield, and adverse events. The acquisition rate of adequate specimens and diagnostic accuracy were compared according to EUS-FNB techniques.
Results
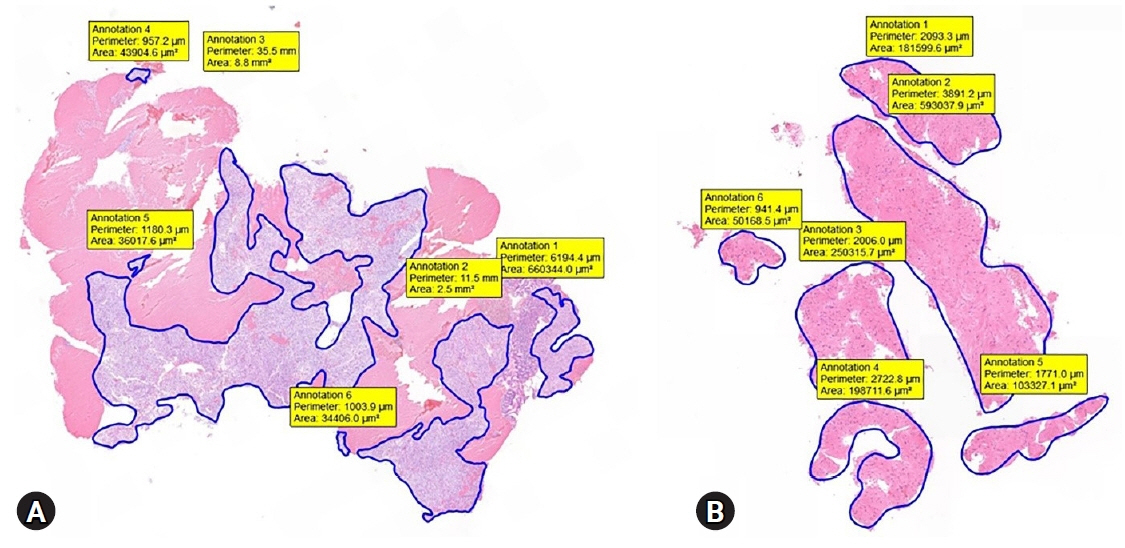
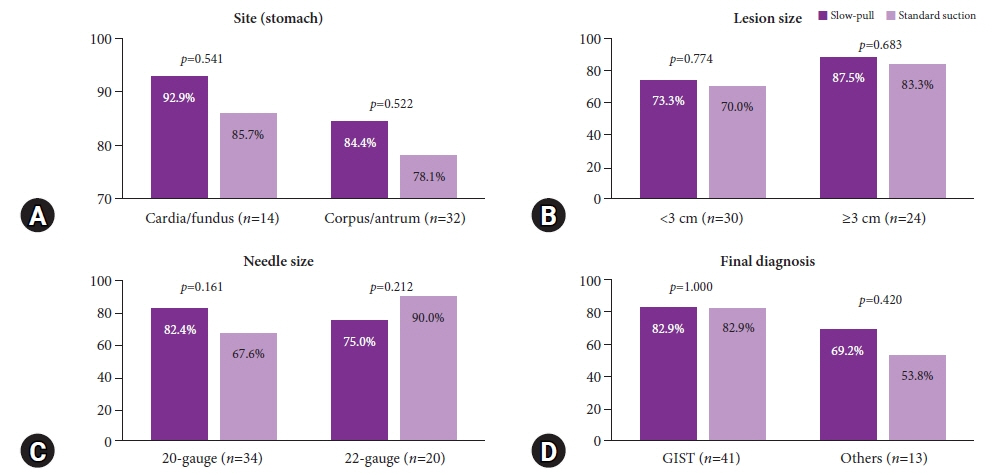
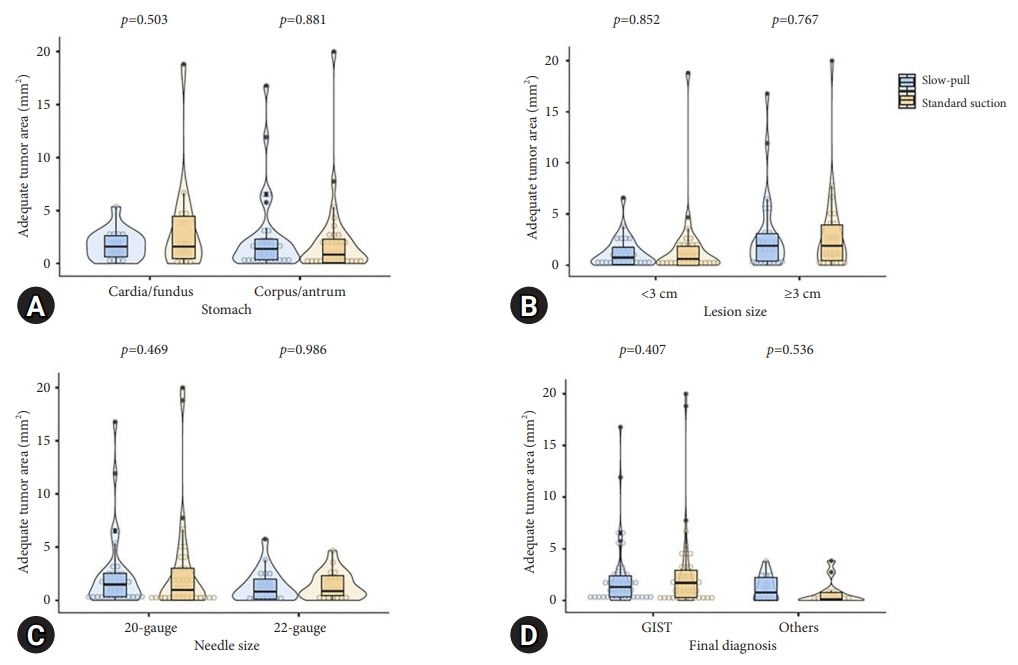
The mean lesion size was 42.6±36.4 mm, and most patients were diagnosed with GI stromal tumor (75.9%). The overall diagnostic accuracies of the SP and SS techniques were 83.3% and 81.5%, respectively (p=0.800). The rates of obtaining adequate core tissue were 79.6% and 75.9%, respectively (p=0.799). No significant clinical factors affected the rate of obtaining adequate core tissue, including lesion location and size, FNB needle size, and final diagnosis.
Conclusions
SP and SS had comparable diagnostic accuracies and adequate core tissue acquisition for GI SETs via EUS-FNB.
Keyword
Figure
Cited by 1 articles
-
Prevalence, natural progression, and clinical practices of upper gastrointestinal subepithelial lesions in Korea: a multicenter study
Younghee Choe, Yu Kyung Cho, Gwang Ha Kim, Jun-Ho Choi, Eun Soo Kim, Ji Hyun Kim, Eun Kwang Choi, Tae Hyeon Kim, Seong-Hun Kim, Do Hoon Kim
Clin Endosc. 2023;56(6):744-753. doi: 10.5946/ce.2023.005.
Reference
-
1. Dumonceau JM, Deprez PH, Jenssen C, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline-updated January 2017. Endoscopy. 2017; 49:695–714.2. Polkowski M. Endoscopic ultrasound and endoscopic ultrasound-guided fine-needle biopsy for the diagnosis of malignant submucosal tumors. Endoscopy. 2005; 37:635–645.3. Karaca C, Turner BG, Cizginer S, et al. Accuracy of EUS in the evaluation of small gastric subepithelial lesions. Gastrointest Endosc. 2010; 71:722–727.4. Rösch T, Kapfer B, Will U, et al. Endoscopic ultrasonography. Accuracy of endoscopic ultrasonography in upper gastrointestinal submucosal lesions: a prospective multicenter study. Scand J Gastroenterol. 2002; 37:856–862.5. Lee JH, Choi KD, Kim MY, et al. Clinical impact of EUS-guided Trucut biopsy results on decision making for patients with gastric subepithelial tumors ≥2 cm in diameter. Gastrointest Endosc. 2011; 74:1010–1018.6. Mekky MA, Yamao K, Sawaki A, et al. Diagnostic utility of EUS-guided FNA in patients with gastric submucosal tumors. Gastrointest Endosc. 2010; 71:913–919.7. Philipper M, Hollerbach S, Gabbert HE, et al. Prospective comparison of endoscopic ultrasound-guided fine-needle aspiration and surgical histology in upper gastrointestinal submucosal tumors. Endoscopy. 2010; 42:300–305.8. Polkowski M, Bergman JJ. Endoscopic ultrasonography-guided biopsy for submucosal tumors: needless needling? Endoscopy. 2010; 42:324–326.9. Akahoshi K, Sumida Y, Matsui N, et al. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J Gastroenterol. 2007; 13:2077–2082.10. Hoda KM, Rodriguez SA, Faigel DO. EUS-guided sampling of suspected GI stromal tumors. Gastrointest Endosc. 2009; 69:1218–1223.11. Yoshida S, Yamashita K, Yokozawa M, et al. Diagnostic findings of ultrasound-guided fine-needle aspiration cytology for gastrointestinal stromal tumors: proposal of a combined cytology with newly defined features and histology diagnosis. Pathol Int. 2009; 59:712–719.12. Fernandez-Esparrach G, Sendino O, Sole M, et al. Endoscopic ultrasound-guided fine-needle aspiration and trucut biopsy in the diagnosis of gastric stromal tumors: a randomized crossover study. Endoscopy. 2010; 42:292–299.13. Polkowski M, Gerke W, Jarosz D, et al. Diagnostic yield and safety of endoscopic ultrasound-guided trucut biopsy in patients with gastric submucosal tumors: a prospective study. Endoscopy. 2009; 41:329–334.14. de Moura DT, McCarty TR, Jirapinyo P, et al. EUS-guided fine-needle biopsy sampling versus FNA in the diagnosis of subepithelial lesions: a large multicenter study. Gastrointest Endosc. 2020; 92:108–119.15. Facciorusso A, Sunny SP, Del Prete V, et al. Comparison between fine-needle biopsy and fine-needle aspiration for EUS-guided sampling of subepithelial lesions: a meta-analysis. Gastrointest Endosc. 2020; 91:14–22.16. Affolter KE, Schmidt RL, Matynia AP, et al. Needle size has only a limited effect on outcomes in EUS-guided fine needle aspiration: a systematic review and meta-analysis. Dig Dis Sci. 2013; 58:1026–1034.17. Pih GY, Kim DH. Endoscopic ultrasound-guided fine needle aspiration and biopsy in gastrointestinal subepithelial tumors. Clin Endosc. 2019; 52:314–320.18. Tadic M, Stoos-Veic T, Kusec R. Endoscopic ultrasound guided fine needle aspiration and useful ancillary methods. World J Gastroenterol. 2014; 20:14292–14300.19. Varadarajulu S, Fockens P, Hawes RH. Best practices in endoscopic ultrasound-guided fine-needle aspiration. Clin Gastroenterol Hepatol. 2012; 10:697–703.20. Iwashita T, Nakai Y, Samarasena JB, et al. High single-pass diagnostic yield of a new 25-gauge core biopsy needle for EUS-guided FNA biopsy in solid pancreatic lesions. Gastrointest Endosc. 2013; 77:909–915.21. Matsubayashi H, Matsui T, Yabuuchi Y, et al. Endoscopic ultrasonography guided-fine needle aspiration for the diagnosis of solid pancreaticobiliary lesions: clinical aspects to improve the diagnosis. World J Gastroenterol. 2016; 22:628–640.22. Fabbri C, Polifemo AM, Luigiano C, et al. Endoscopic ultrasound-guided fine needle aspiration with 22- and 25-gauge needles in solid pancreatic masses: a prospective comparative study with randomisation of needle sequence. Dig Liver Dis. 2011; 43:647–652.23. Nakai Y, Isayama H, Chang KJ, et al. Slow pull versus suction in endoscopic ultrasound-guided fine-needle aspiration of pancreatic solid masses. Dig Dis Sci. 2014; 59:1578–1585.24. Crino SF, Le Grazie M, Manfrin E, et al. Randomized trial comparing fork-tip and side-fenestrated needles for EUS-guided fine-needle biopsy of solid pancreatic lesions. Gastrointest Endosc. 2020; 92:648–658.25. Crinò SF, Manfrin E, Scarpa A, et al. EUS-FNB with or without on-site evaluation for the diagnosis of solid pancreatic lesions (FROSENOR): protocol for a multicenter randomized non-inferiority trial. Dig Liver Dis. 2019; 51:901–906.26. Zhang XC, Li QL, Yu YF, et al. Diagnostic efficacy of endoscopic ultrasound-guided needle sampling for upper gastrointestinal subepithelial lesions: a meta-analysis. Surg Endosc. 2016; 30:2431–2441.27. Paik WH, Park Y, Park DH, et al. Prospective evaluation of new 22 gauge endoscopic ultrasound core needle using capillary sampling with stylet slow-pull technique for intra-abdominal solid masses. J Clin Gastroenterol. 2015; 49:199–205.28. Antonini F, Delconte G, Fuccio L, et al. EUS-guided tissue sampling with a 20-gauge core biopsy needle for the characterization of gastrointestinal subepithelial lesions: a multicenter study. Endosc Ultrasound. 2019; 8:105–110.29. Kim DH, Kim GH, Cho CM, et al. Feasibility of a 20-gauge ProCore needle in EUS-guided subepithelial tumor sampling: a prospective multicenter study. BMC Gastroenterol. 2018; 18:151.30. Bang JY, Hebert-Magee S, Hasan MK, et al. Endoscopic ultrasonography-guided biopsy using a Franseen needle design: initial assessment. Dig Endosc. 2017; 29:338–346.31. Schlag C, Menzel C, Götzberger M, et al. Endoscopic ultrasound-guided tissue sampling of small subepithelial tumors of the upper gastrointestinal tract with a 22-gauge core biopsy needle. Endosc Int Open. 2017; 5:E165–E171.32. Yi M, Xia L, Zhou Y, et al. Prognostic value of tumor necrosis in gastrointestinal stromal tumor: a meta-analysis. Medicine (Baltimore). 2019; 98:e15338.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endoscopic Ultrasound-Guided Fine Needle Aspiration and Biopsy in Gastrointestinal Subepithelial Tumors
- Endoscopic Ultrasound-Fine Needle Aspiration versus Core Biopsy for the Diagnosis of Subepithelial Tumors
- Slow-Pull Using a Fanning Technique Is More Useful Than the Standard Suction Technique in EUS-Guided Fine Needle Aspiration in Pancreatic Masses
- How to optimize the diagnostic yield of endoscopic ultrasoundguided fine-needle sampling in solid pancreatic lesions from a technical perspective
- Endoscopic Ultrasound-Guided Fine Needle Aspiration in Submucosal Lesion