Clin Endosc.
2022 Sep;55(5):615-625. 10.5946/ce.2022.133.
Mucosal incision-assisted biopsy versus endoscopic ultrasound-assisted tissue acquisition for subepithelial lesions: a systematic review and meta-analysis
- Affiliations
-
- 1Nizam's Institute of Medical Sciences, Hyderabad, India
- 2Institute of Gastrosciences and Liver, Apollo Multispecialty Hospital, Kolkata, India
- 3Department of Digestive Diseases and Clinical Nutrition, Tata Memorial Hospital, Mumbai, India
- KMID: 2533299
- DOI: http://doi.org/10.5946/ce.2022.133
Abstract
- Background/Aims
Mucosal incision-assisted biopsy (MIAB) for tissue acquisition (TA) from subepithelial lesions (SELs) is emerging as an alternative to endoscopic ultrasound (EUS)-guided TA. Only a limited number of studies compared the diagnostic utility of MIAB and EUS for upper gastrointestinal (GI) SELs; therefore, we conducted this systematic review and meta-analysis.
Methods
A comprehensive literature search from January 2020 to January 2022 was performed to compare the diagnostic accuracy and safety of MIAB and EUS-guided TA for upper GI SELs.
Results
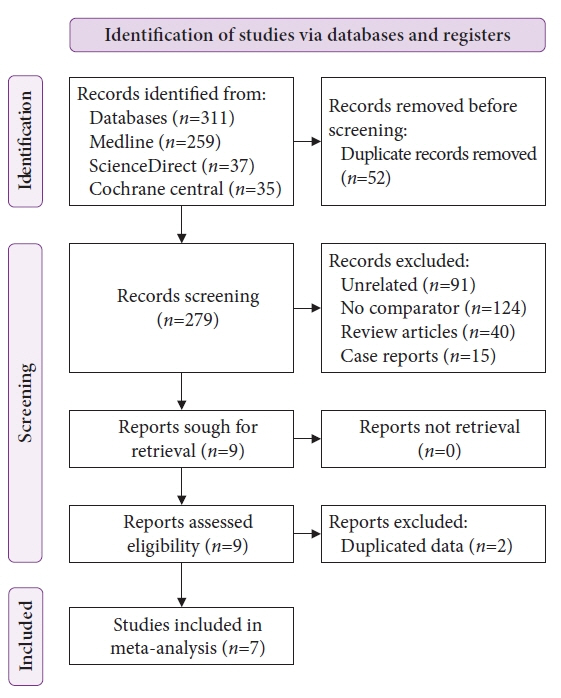
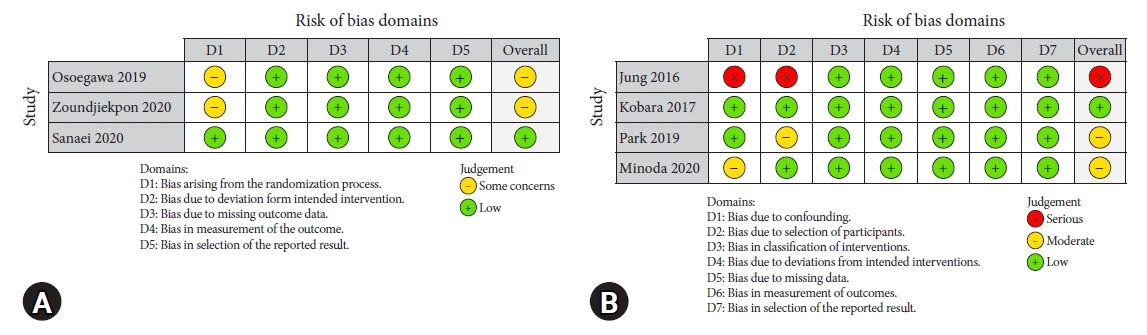
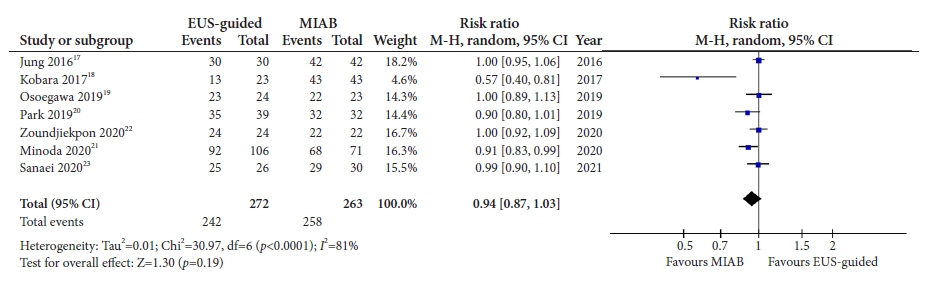
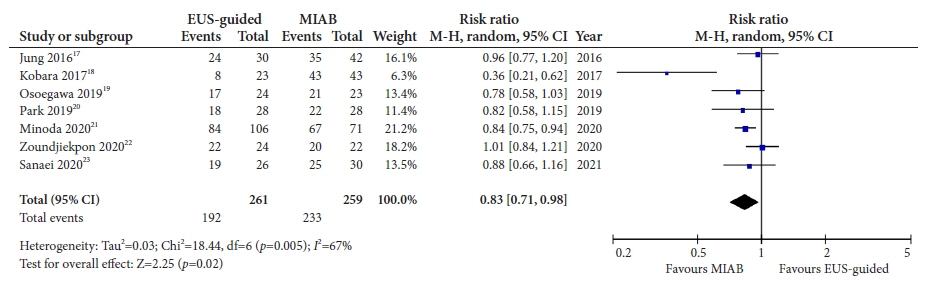
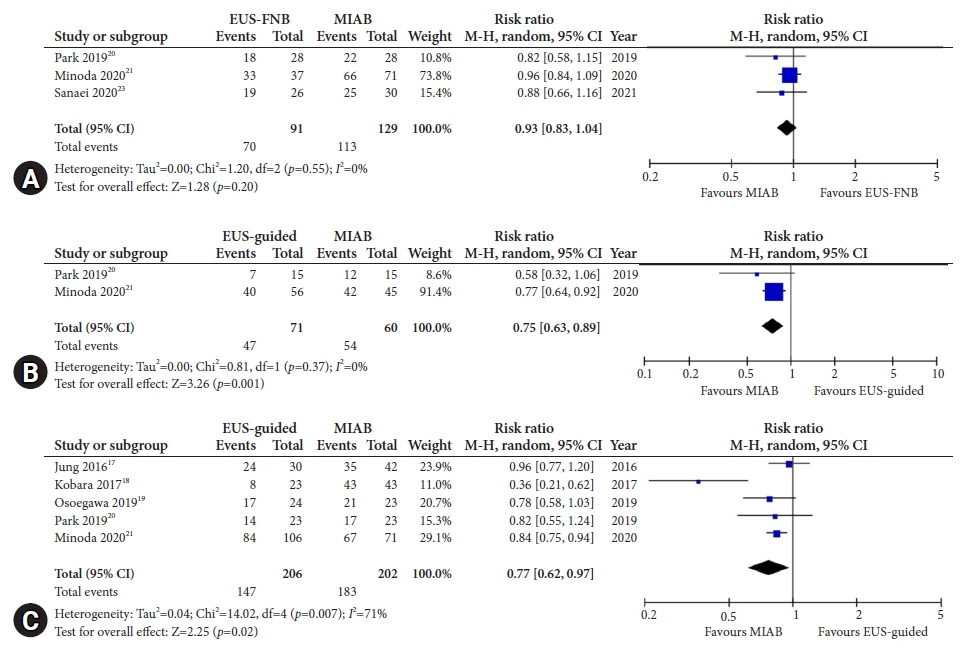
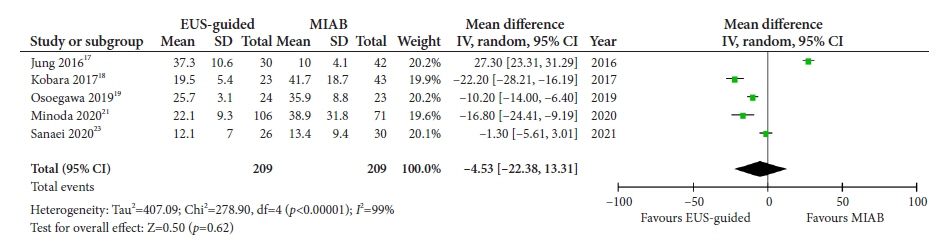
Seven studies were included in this meta-analysis. The pooled technical success rate (risk ratio [RR], 0.96; 95% confidence interval [CI], 0.89–1.04) and procedural time (mean difference=–4.53 seconds; 95% CI, –22.38 to 13.31] were comparable between both the groups. The overall chance of obtaining a positive diagnostic yield was lower with EUS than with MIAB for all lesions (RR, 0.83; 95% CI, 0.71–0.98) but comparable when using a fine-needle biopsy needle (RR, 0.93; 95% CI, 0.83–1.04). The positive diagnostic yield of MIAB was higher for lesions <20 mm (RR, 0.75; 95% CI, 0.63–0.89). Six studies reported no adverse events.
Conclusions
MIAB can be considered an effective alternative to EUS-guided TA for upper GI SELs without an increased risk of adverse events.
Figure
Reference
-
1. Humphris JL, Jones DB. Subepithelial mass lesions in the upper gastrointestinal tract. J Gastroenterol Hepatol. 2008; 23:556–566.2. Hawes R, Fockens P, Varadarajulu S. Endosonography. 4th ed. Philadelphia: Elsevier;2018.3. Polkowski M. Endoscopic ultrasound and endoscopic ultrasound-guided fine-needle biopsy for the diagnosis of malignant submucosal tumors. Endoscopy. 2005; 37:635–645.4. Buscaglia JM, Nagula S, Jayaraman V, et al. Diagnostic yield and safety of jumbo biopsy forceps in patients with subepithelial lesions of the upper and lower GI tract. Gastrointest Endosc. 2012; 75:1147–1152.5. Akahoshi K, Sumida Y, Matsui N, et al. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J Gastroenterol. 2007; 13:2077–2082.6. Attila T, Aydın Ö. Lesion size determines diagnostic yield of EUS-FNA with onsite cytopathologic evaluation for upper gastrointestinal subepithelial lesions. Turk J Gastroenterol. 2018; 29:436–441.7. van Riet PA, Erler NS, Bruno MJ, et al. Comparison of fine-needle aspiration and fine-needle biopsy devices for endoscopic ultrasound-guided sampling of solid lesions: a systemic review and meta-analysis. Endoscopy. 2021; 53:411–423.8. Facciorusso A, Sunny SP, Del Prete V, et al. Comparison between fine-needle biopsy and fine-needle aspiration for EUS-guided sampling of subepithelial lesions: a meta-analysis. Gastrointest Endosc. 2020; 91:14–22.9. Zhang XC, Li QL, Yu YF, et al. Diagnostic efficacy of endoscopic ultrasound-guided needle sampling for upper gastrointestinal subepithelial lesions: a meta-analysis. Surg Endosc. 2016; 30:2431–2441.10. Yokohata N, Tamegai Y, Tokuhara M, et al. 3 case of gastric SMT (submucal tumor) which was diagnoced before operation: open biopsy with ESD (endoscopic submucal dissection) for interstind SMT. Prog Dig Endosc. 2007; 70:82–83.11. Ihara E, Matsuzaka H, Honda K, et al. Mucosal-incision assisted biopsy for suspected gastric gastrointestinal stromal tumors. World J Gastrointest Endosc. 2013; 5:191–196.12. Dhaliwal A, Kolli S, Dhindsa BS, et al. Clinical efficacy and safety of mucosal incision-assisted biopsy for the diagnosis of upper gastrointestinal subepithelial tumors: a systematic review and meta-analysis. Ann Gastroenterol. 2020; 33:155–161.13. Dhaliwal A, Kolli S, Dhindsa BS, et al. Diagnostic yield of deep biopsy via endoscopic submucosal dissection for the diagnosis of upper gastrointestinal subepithelial tumors: a systematic review and meta-analysis. Ann Gastroenterol. 2020; 33:30–37.14. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021; 372:n71.15. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928.16. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016; 355:i4919.17. Jung YS, Lee H, Kim K, et al. Using forceps biopsy after small submucosal dissection in the diagnosis of gastric subepithelial tumors. J Korean Med Sci. 2016; 31:1768–1774.18. Kobara H, Mori H, Nishimoto N, et al. Comparison of submucosal tunneling biopsy versus EUS-guided FNA for gastric subepithelial lesions: a prospective study with crossover design. Endosc Int Open. 2017; 5:E695–E705.19. Osoegawa T, Minoda Y, Ihara E, et al. Mucosal incision-assisted biopsy versus endoscopic ultrasound-guided fine-needle aspiration with a rapid on-site evaluation for gastric subepithelial lesions: a randomized cross-over study. Dig Endosc. 2019; 31:413–421.20. Park J, Park JC, Jo JH, et al. Prospective comparative study of endoscopic ultrasonography-guided fine-needle biopsy and unroofing biopsy. Dig Liver Dis. 2019; 51:831–836.21. Minoda Y, Chinen T, Osoegawa T, et al. Superiority of mucosal incision-assisted biopsy over ultrasound-guided fine needle aspiration biopsy in diagnosing small gastric subepithelial lesions: a propensity score matching analysis. BMC Gastroenterol. 2020; 20:19.22. Zoundjiekpon V, Falt P, Fojtik P, et al. Endosonography-guided fine-needle aspiration versus “Key-Hole Biopsy” in the diagnostics of upper gastrointestinal subepithelial tumors: a prospective randomized interventional study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2020; 164:63–70.23. Sanaei O, Fernández-Esparrach G, De La Serna-Higuera C, et al. EUS-guided 22-gauge fine needle biopsy versus single-incision with needle knife for the diagnosis of upper gastrointestinal subepithelial lesions: a randomized controlled trial. Endosc Int Open. 2020; 8:E266–E273.24. Deprez PH, Moons LM, OʼToole D, et al. Endoscopic management of subepithelial lesions including neuroendocrine neoplasms: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022; 54:412–429.25. Casali PG, Blay JY, Abecassis N, et al. Gastrointestinal stromal tumours: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2022; 33:20–33.26. Nishida T, Hirota S, Yanagisawa A, et al. Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int J Clin Oncol. 2008; 13:416–430.27. Kim HH. Endoscopic treatment for gastrointestinal stromal tumor: advantages and hurdles. World J Gastrointest Endosc. 2015; 7:192–205.28. Facciorusso A, Crinò SF, Ramai D, et al. Comparison between endoscopic ultrasound-guided fine-needle biopsy and bite-on-bite jumbo biopsy for sampling of subepithelial lesions. Dig Liver Dis. 2022; 54:676–683.29. Larghi A, Fuccio L, Chiarello G, et al. Fine-needle tissue acquisition from subepithelial lesions using a forward-viewing linear echoendoscope. Endoscopy. 2014; 46:39–45.30. Matsuzaki I, Miyahara R, Hirooka Y, et al. Forward-viewing versus oblique-viewing echoendoscopes in the diagnosis of upper GI subepithelial lesions with EUS-guided FNA: a prospective, randomized, crossover study. Gastrointest Endosc. 2015; 82:287–295.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endoscopic Management of Gastric Subepithelial Tumor
- Endoscopic Ultrasound-Fine Needle Aspiration versus Core Biopsy for the Diagnosis of Subepithelial Tumors
- Advancements in the Diagnosis of Gastric Subepithelial Tumors
- Mucosal Incision-Assisted Endoscopic Biopsy as an Alternative to Endoscopic Ultrasound-Guided Fine-Needle Aspiration/Biopsy for Gastric Subepithelial Tumor
- Advances in the Management of Upper Gastrointestinal Subepithelial Tumor: Pathologic Diagnosis Using Endoscopy without Endoscopic Ultrasound-Guided Biopsy