Clin Exp Otorhinolaryngol.
2022 Aug;15(3):220-229. 10.21053/ceo.2022.00038.
Hyaluronan Synthase 1: A Novel Candidate Gene Associated With Late-Onset Non-syndromic Hereditary Hearing Loss
- Affiliations
-
- 1Department of Otolaryngology-Head and Neck Surgery, Chonnam National University Hospital, Chonnam National University Medical School, Gwangju, Korea
- 2Department of Biomedical Science, College of Medicine, Chonnam National University Graduate School, BK21 PLUS Center for Creative Biomedical Scientists at Chonnam National University, Gwangju, Korea
- 3Hospital-based Business Innovation Center, Chonnam National University Hospital, Gwangju, Korea
- 4First ENT Clinic, Gwangju, Korea
- KMID: 2532986
- DOI: http://doi.org/10.21053/ceo.2022.00038
Abstract
Objectives
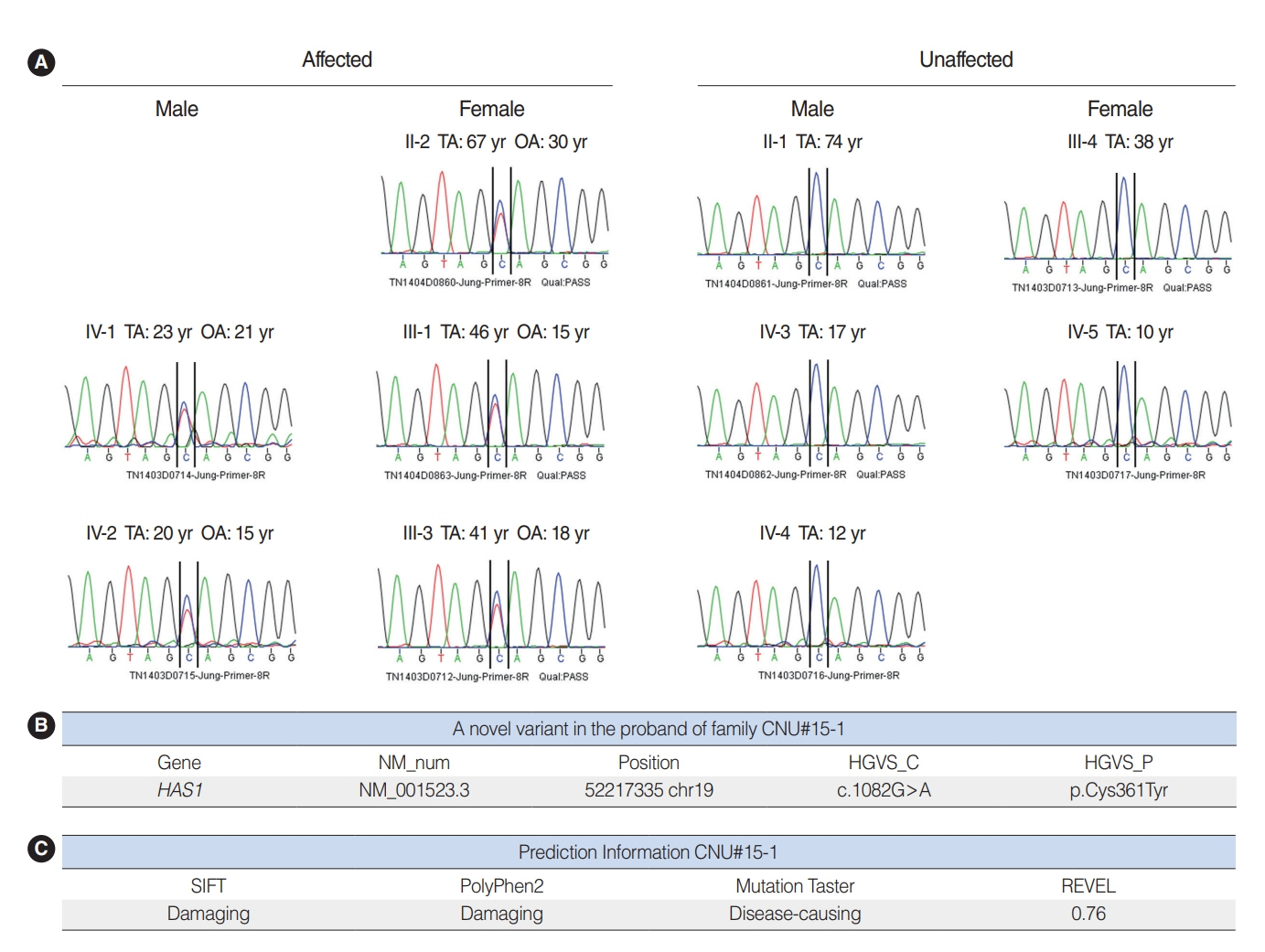
. Hyaluronan synthase 1 (HAS1) is a membrane-bound protein that is abundant in the epidermis and dermis, and it is important for skin function. However, its association with hearing loss has not yet been studied. Herein, we sought to evaluate the potential contribution of HAS1: c.1082G>A to genetic hearing loss.
Methods
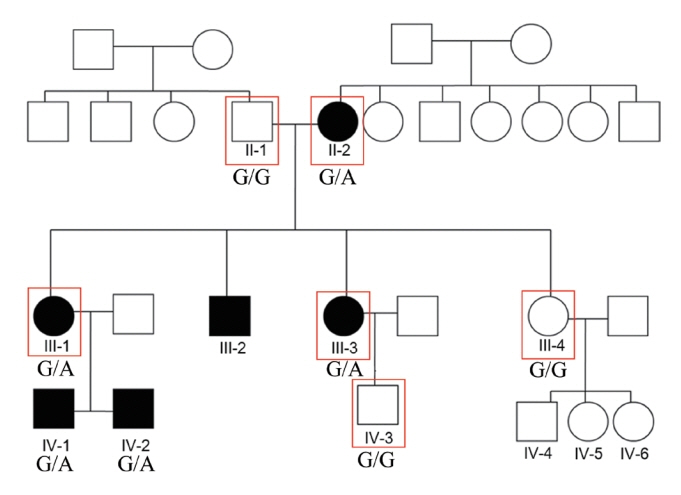
. We used whole-exome sequencing to analyze blood DNA samples of six patients of a family with autosomal dominant familial late-onset progressive hearing loss, which was revealed to be related to a variant of the HAS1 gene. Confirmatory Sanger sequencing was performed with samples from 10 members. A missense variant was detected in HAS1 (c.1082 G>A, p.Cys361Tyr). In silico analyses predicted this variant to result in the functional loss of HAS1. Immunostaining was conducted using wild-type mouse samples to verify HAS1 expression.
Results
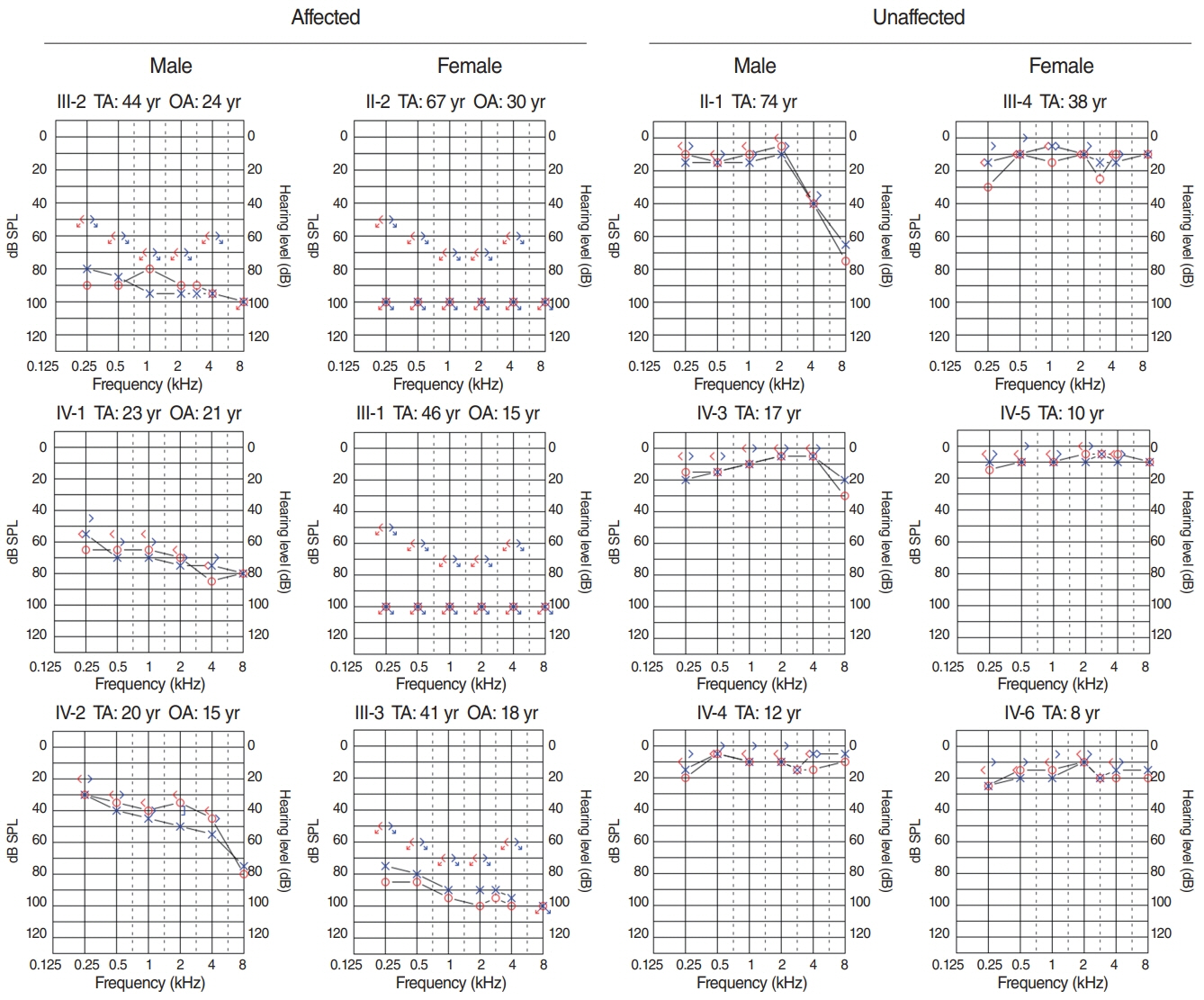
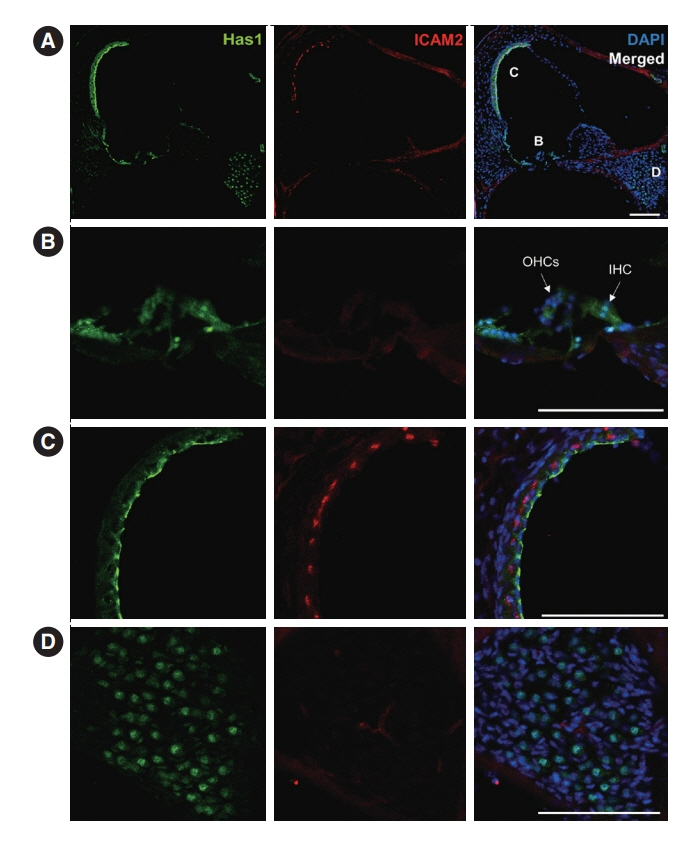
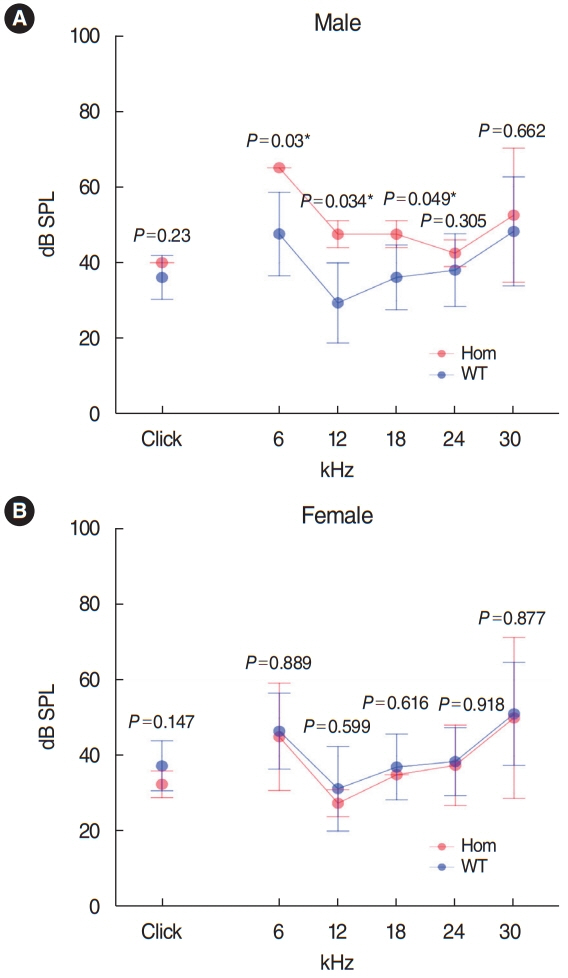
. Has1 was detected in an otocyst at E10.5. In the pup, Has1 expression was localized in the stria vascularis (SV), hair cells, supporting cells of the organ of Corti, and some spiral ganglion neurons. SV marginal cells markedly expressed Has1 in the adult stage. The hearing threshold in the Has1-depleted condition was investigated by accessing the International Mouse Phenotyping Consortium’s Auditory Brainstem Response (ABR) data. ABR of Has1 knock-out mice showed threshold elevations at 6, 12, and 18 kHz in young male adults.
Conclusion
. HAS1 may have a close relationship with auditory function and genetic hearing loss. Further investigation is needed to reveal the precise role of HAS1 in the auditory system. HAS1 is a candidate gene for future hereditary hearing loss genetic testing.
Keyword
Figure
Reference
-
1. Friedman TB, Griffith AJ. Human nonsyndromic sensorineural deafness. Annu Rev Genomics Hum Genet. 2003; Sep. 4:341–402.2. Stelma F, Bhutta MF. Non-syndromic hereditary sensorineural hearing loss: review of the genes involved. J Laryngol Otol. 2014; Jan. 128(1):13–21.3. Torronen K, Nikunen K, Karna R, Tammi M, Tammi R, Rilla K. Tissue distribution and subcellular localization of hyaluronan synthase isoenzymes. Histochem Cell Biol. 2014; Jan. 141(1):17–31.4. Itano N, Sawai T, Yoshida M, Lenas P, Yamada Y, Imagawa M, et al. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J Biol Chem. 1999; Aug. 274(35):25085–92.5. Dickinson ME, Flenniken AM, Ji X, Teboul L, Wong MD, White JK, et al. High-throughput discovery of novel developmental phenotypes. Nature. 2016; Sep. 537(7621):508–14.6. Petersen MB. Non-syndromic autosomal-dominant deafness. Clin Genet. 2002; Jul. 62(1):1–13.7. Han HM, Kwak JW, Kim HG, Lee H, Kim YC, Park E, et al. Nationwide analysis of mortality rates and related surgical procedures in hearing disability patients in South Korea. J Audiol Otol. 2020; Oct. 24(4):204–9.8. Pattisapu P, Lindquist NR, Appelbaum EN, Silva RC, Vrabec JT, Sweeney AD. A systematic review of cochlear implant outcomes in prelingually-deafened, late-implanted patients. Otol Neurotol. 2020; Apr. 41(4):444–51.9. Wang L, Kempton JB, Brigande JV. Gene therapy in mouse models of deafness and balance dysfunction. Front Mol Neurosci. 2018; Aug. 29(11):300.10. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015; May. 17(5):405–24.11. Oza AM, DiStefano MT, Hemphill SE, Cushman BJ, Grant AR, Siegert RK, et al. Expert specification of the ACMG/AMP variant interpretation guidelines for genetic hearing loss. Hum Mutat. 2018; Nov. 39(11):1593–613.12. Holmes MW, Bayliss MT, Muir H. Hyaluronic acid in human articular cartilage: age-related changes in content and size. Biochem J. 1988; Mar. 250(2):435–41.13. Averbeck M, Gebhardt CA, Voigt S, Beilharz S, Anderegg U, Termeer CC, et al. Differential regulation of hyaluronan metabolism in the epidermal and dermal compartments of human skin by UVB irradiation. J Invest Dermatol. 2007; Mar. 127(3):687–97.14. Hall CL, Wang C, Lange LA, Turley EA. Hyaluronan and the hyaluronan receptor RHAMM promote focal adhesion turnover and transient tyrosine kinase activity. J Cell Biol. 1994; Jul. 126(2):575–88.15. Zhang S, Chang MC, Zylka D, Turley S, Harrison R, Turley EA. The hyaluronan receptor RHAMM regulates extracellular-regulated kinase. J Biol Chem. 1998; May. 273(18):11342–8.16. Knudson CB, Knudson W. Hyaluronan-binding proteins in development, tissue homeostasis, and disease. FASEB J. 1993; Oct. 7(13):1233–41.17. Sherman L, Sleeman J, Herrlich P, Ponta H. Hyaluronate receptors: key players in growth, differentiation, migration and tumor progression. Curr Opin Cell Biol. 1994; Oct. 6(5):726–33.18. Wu RL, Sedlmeier G, Kyjacova L, Schmaus A, Philipp J, Thiele W, et al. Hyaluronic acid-CD44 interactions promote BMP4/7-dependent Id1/3 expression in melanoma cells. Sci Rep. 2018; Oct. 8(1):14913.19. Tammi MI, Oikari S, Pasonen-Seppanen S, Rilla K, Auvinen P, Tammi RH. Activated hyaluronan metabolism in the tumor matrix: causes and consequences. Matrix Biol. 2019; May. 78-79:147–64.20. Stern R. Hyaluronan catabolism: a new metabolic pathway. Eur J Cell Biol. 2004; Aug. 83(7):317–25.21. Watanabe K, Yamaguchi Y. Molecular identification of a putative human hyaluronan synthase. J Biol Chem. 1996; Sep. 271(38):22945–8.22. Itano N, Kimata K. Molecular cloning of human hyaluronan synthase. Biochem Biophys Res Commun. 1996; May. 222(3):816–20.23. Shyjan AM, Heldin P, Butcher EC, Yoshino T, Briskin MJ. Functional cloning of the cDNA for a human hyaluronan synthase. J Biol Chem. 1996; Sep. 271(38):23395–9.24. Spicer AP, Augustine ML, McDonald JA. Molecular cloning and characterization of a putative mouse hyaluronan synthase. J Biol Chem. 1996; Sep. 271(38):23400–6.25. Spicer AP, Olson JS, McDonald JA. Molecular cloning and characterization of a cDNA encoding the third putative mammalian hyaluronan synthase. J Biol Chem. 1997; Apr. 272(14):8957–61.26. Spicer AP, Seldin MF, Olsen AS, Brown N, Wells DE, Doggett NA, et al. Chromosomal localization of the human and mouse hyaluronan synthase genes. Genomics. 1997; May. 41(3):493–7.27. Rilla K, Oikari S, Jokela TA, Hyttinen JM, Karna R, Tammi RH, et al. Hyaluronan synthase 1 (HAS1) requires higher cellular UDP-GlcNAc concentration than HAS2 and HAS3. J Biol Chem. 2013; Feb. 288(8):5973–83.28. Kultti A, Karna R, Rilla K, Nurminen P, Koli E, Makkonen KM, et al. Methyl-beta-cyclodextrin suppresses hyaluronan synthesis by down-regulation of hyaluronan synthase 2 through inhibition of Akt. J Biol Chem. 2010; Jul. 285(30):22901–10.29. Vigetti D, Genasetti A, Karousou E, Viola M, Moretto P, Clerici M, et al. Proinflammatory cytokines induce hyaluronan synthesis and monocyte adhesion in human endothelial cells through hyaluronan synthase 2 (HAS2) and the nuclear factor-kappaB (NF-kappaB) pathway. J Biol Chem. 2010; Aug. 285(32):24639–45.30. Chow G, Tauler J, Mulshine JL. Cytokines and growth factors stimulate hyaluronan production: role of hyaluronan in epithelial to mesenchymal-like transition in non-small cell lung cancer. J Biomed Biotechnol. 2010; 2010:485468.31. Akazawa Y, Sayo T, Sugiyama Y, Sato T, Akimoto N, Ito A, et al. Adiponectin resides in mouse skin and upregulates hyaluronan synthesis in dermal fibroblasts. Connect Tissue Res. 2011; 52(4):322–8.32. Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A Jr, et al. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000; Aug. 106(3):349–60.33. Bai KJ, Spicer AP, Mascarenhas MM, Yu L, Ochoa CD, Garg HG, et al. The role of hyaluronan synthase 3 in ventilator-induced lung injury. Am J Respir Crit Care Med. 2005; Jul. 172(1):92–8.34. Kobayashi N, Miyoshi S, Mikami T, Koyama H, Kitazawa M, Takeoka M, et al. Hyaluronan deficiency in tumor stroma impairs macrophage trafficking and tumor neovascularization. Cancer Res. 2010; Sep. 70(18):7073–83.35. Kriangkum J, Warkentin A, Belch AR, Pilarski LM. Alteration of introns in a hyaluronan synthase 1 (HAS1) minigene convert PremRNA [corrected] splicing to the aberrant pattern in multiple myeloma (MM): MM patients harbor similar changes. PLoS One. 2013; Jan. 8(1):e53469.36. Golshani R, Hautmann SH, Estrella V, Cohen BL, Kyle CC, Manoharan M, et al. HAS1 expression in bladder cancer and its relation to urinary HA test. Int J Cancer. 2007; Apr. 120(8):1712–20.37. Auvinen P, Rilla K, Tumelius R, Tammi M, Sironen R, Soini Y, et al. Hyaluronan synthases (HAS1-3) in stromal and malignant cells correlate with breast cancer grade and predict patient survival. Breast Cancer Res Treat. 2014; Jan. 143(2):277–86.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Genetic Background of Congenital Hearing Loss
- A 250-kb Microdeletion Identified in Chromosome 16 Is Associated With Non-Syndromic Sensorineural Hearing Loss in a South Indian Consanguineous Family
- Genetic Hearing Loss and Gene Therapy
- Etiology of Hearing Loss and Genetic Hearing Loss
- Molecular Genetic Analysis of Connexin 26 in Korean Congenital Hearing Loss