Int J Stem Cells.
2022 Aug;15(3):233-246. 10.15283/ijsc21158.
Lupus Heart Disease Modeling with Combination of Induced Pluripotent Stem Cell-Derived Cardiomyocytes and Lupus Patient Serum
- Affiliations
-
- 1CiSTEM Laboratory, Convergent Research Consortium for Immunologic Disease, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2YiPSCELL, 47-3, Banpo-dearo 39-gil, Seocho-gu, Seoul, Korea
- 3Division of Rheumatology, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2532398
- DOI: http://doi.org/10.15283/ijsc21158
Abstract
- Background and Objectives
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease mainly affecting young women of childbearing age. SLE affects the skin, joints, muscles, kidneys, lungs, and heart. Cardiovascular complications are common causes of death in patients with SLE. However, the complexity of the cardiovascular system and the rarity of SLE make it difficult to investigate these morbidities. Patient-derived induced pluripotent stem cells (iPSCs) serve as a novel tool for drug screening and pathophysiological studies in the absence of patient samples.
Methods and Results
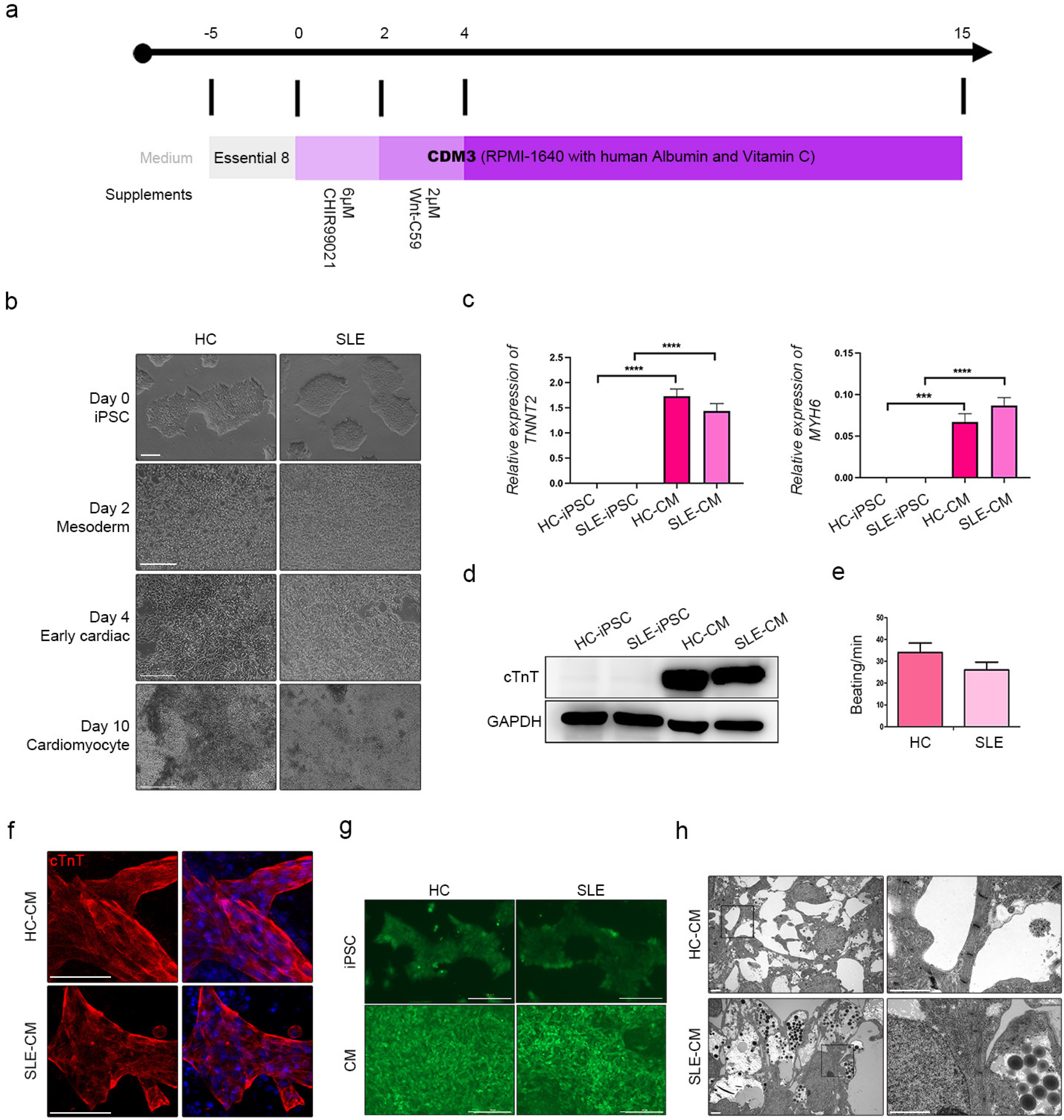
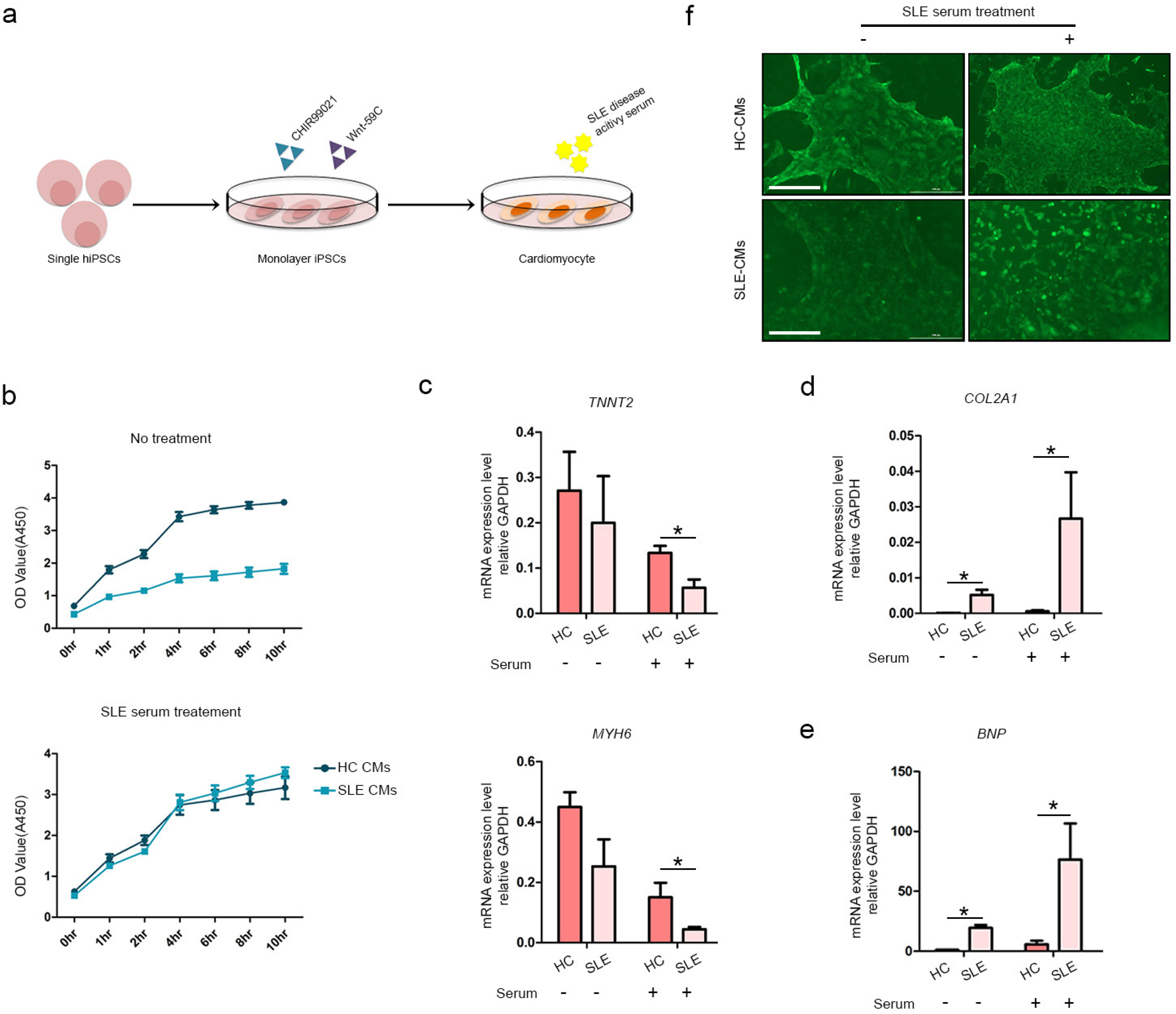
We differentiated CMs from HC- and SLE-iPSCs using 2D culture platforms. SLE-CMs showed decreased proliferation and increased levels of fibrosis and hypertrophy marker expression; however, HC-and SLE-monolayer CMs reacted differently to SLE serum treatment. HC-iPSCs were also differentiated into CMs using 3D spheroid culture and anti-Ro autoantibody was treated along with SLE serum. 3D-HC-CMs generated more mature CMs compared to the CMs generated using 2D culture. The treatment of anti-Ro autoantibody rapidly increased the gene expression of fibrosis, hypertrophy, and apoptosis markers, and altered the calcium signaling in the CMs.
Conclusions
iPSC derived cardiomyocytes with patient-derived serum, and anti-Ro antibody treatment could serve in effective autoimmune disease modeling including SLE. We believe that the present study might briefly provide possibilities on the application of a combination of patient-derived materials and iPSCs in disease modeling of autoimmune diseases.
Figure
Reference
-
References
1. Nikdoust F, Bolouri E, Tabatabaei SA, Goudarzvand M, Faezi ST. 2018; Early diagnosis of cardiac involvement in systemic lupus erythematosus via global longitudinal strain (GLS) by speckle tracking echocardiography. J Cardiovasc Thorac Res. 10:231–235. DOI: 10.15171/jcvtr.2018.40. PMID: 30680083. PMCID: PMC6335988. PMID: d271d3c635cf429aa40bc1438d6baac9.2. Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel-Aty H, Gutberlet M, Prasad S, Aletras A, Laissy JP, Paterson I, Filipchuk NG, Kumar A, Pauschinger M, Liu P. 2009; Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol. 53:1475–1487. DOI: 10.1016/j.jacc.2009.02.007. PMID: 19389557. PMCID: PMC2743893. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=64649106481&origin=inward.3. Fors Nieves CE, Izmirly PM. 2016; Mortality in systemic lupus erythematosus: an updated review. Curr Rheumatol Rep. 18:21. DOI: 10.1007/s11926-016-0571-2. PMID: 26984805. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84961575286&origin=inward.4. Bjornadal L, Yin L, Granath F, Klareskog L, Ekbom A. 2004; Cardiovascular disease a hazard despite improved prognosis in patients with systemic lupus erythematosus: results from a Swedish population based study 1964-95. J Rheumatol. 31:713–719. PMID: 15088296. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=1842530440&origin=inward.5. Dhakal BP, Kim CH, Al-Kindi SG, Oliveira GH. 2018; Heart failure in systemic lupus erythematosus. Trends Cardiovasc Med. 28:187–197. DOI: 10.1016/j.tcm.2017.08.015. PMID: 28927572. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85029499397&origin=inward.6. Petrackova A, Smrzova A, Gajdos P, Schubertova M, Schneiderova P, Kromer P, Snasel V, Skacelova M, Mrazek F, Zadrazil J, Horak P, Kriegova E. 2017; Serum protein pattern associated with organ damage and lupus nephritis in systemic lupus erythematosus revealed by PEA immunoassay. Clin Proteomics. 14:32. DOI: 10.1186/s12014-017-9167-8. PMID: 29026368. PMCID: PMC5627398. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85030325753&origin=inward.7. Yoshimi R, Ueda A, Ozato K, Ishigatsubo Y. 2012; Clinical and pathological roles of Ro/SSA autoantibody system. Clin Dev Immunol. 2012:606195. DOI: 10.1155/2012/606195. PMID: 23304190. PMCID: PMC3523155. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84871486502&origin=inward.8. Joo H, Coquery C, Xue Y, Gayet I, Dillon SR, Punaro M, Zurawski G, Banchereau J, Pascual V, Oh S. 2012; Serum from patients with SLE instructs monocytes to promote IgG and IgA plasmablast differentiation. J Exp Med. 209:1335–1348. DOI: 10.1084/jem.20111644. PMID: 22689824. PMCID: PMC3405503. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84864296905&origin=inward.9. Kobayashi R, Mii S, Nakano T, Harada H, Eto H. 2009; Neonatal lupus erythematosus in Japan: a review of the literature. Autoimmun Rev. 8:462–466. DOI: 10.1016/j.autrev.2008.12.013. PMID: 19162245. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=65549101431&origin=inward.10. Prince HE, Hogrefe WR. 1998; Evaluation of a line immunoblot assay for detection of antibodies recognizing extractable nuclear antigens. J Clin Lab Anal. 12:320–324. DOI: 10.1002/(SICI)1098-2825(1998)12:5<320::AID-JCLA13>3.0.CO;2-X. PMID: 9773966. PMCID: PMC6807698.11. Hochberg MC. 1997; Updating the American College of Rheuma-tology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 40:1725. DOI: 10.1002/art.1780400928. PMID: 9324032. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0031229830&origin=inward.12. Deng JS, Bair LW Jr, Shen-Schwarz S, Ramsey-Goldman R, Medsger T Jr. 1987; Localization of Ro (SS-A) antigen in the cardiac conduction system. Arthritis Rheum. 30:1232–1238. DOI: 10.1002/art.1780301105. PMID: 3318846. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0023491372&origin=inward.13. Tseng CE, Buyon JP. 1997; Neonatal lupus syndromes. Rheum Dis Clin North Am. 23:31–54. DOI: 10.1016/S0889-857X(05)70313-6. PMID: 17499707. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0031058139&origin=inward.14. Stea EA, Routsias JG, Clancy RM, Buyon JP, Moutsopoulos HM, Tzioufas AG. 2006; Anti-La/SSB antiidiotypic antibodies in maternal serum: a marker of low risk for neonatal lupus in an offspring. Arthritis Rheum. 54:2228–2234. DOI: 10.1002/art.21954. PMID: 16802359. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33745925881&origin=inward.15. Smith MA, Henault J, Karnell JL, Parker ML, Riggs JM, Sinibaldi D, Taylor DK, Ettinger R, Grant EP, Sanjuan MA, Kolbeck R, Petri MA, Casey KA. 2019; SLE plasma profiling identifies unique signatures of lupus nephritis and discoid lupus. Sci Rep. 9:14433. DOI: 10.1038/s41598-019-50231-y. PMID: 31594956. PMCID: PMC6783423. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85073054574&origin=inward.16. Steiman AJ, Gladman DD, Ibañez D, Urowitz MB. 2010; Prolonged serologically active clinically quiescent systemic lupus erythematosus: frequency and outcome. J Rheumatol. 37:1822–1827. DOI: 10.3899/jrheum.100007. PMID: 20595281. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=77956479892&origin=inward.17. Rim YA, Nam Y, Park N, Jung H, Jang Y, Lee J, Ju JH. 2018; Different chondrogenic potential among human induced pluripotent stem cells from diverse origin primary cells. Stem Cells Int. 2018:9432616. DOI: 10.1155/2018/9432616. PMID: 29535785. PMCID: PMC5828428. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85041850510&origin=inward.18. Shi Y, Inoue H, Wu JC, Yamanaka S. 2017; Induced pluripotent stem cell technology: a decade of progress. Nat Rev Drug Discov. 16:115–130. DOI: 10.1038/nrd.2016.245. PMID: 27980341. PMCID: PMC6416143. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85006285022&origin=inward.19. Li C, Chen S, Zhou Y, Zhao Y, Liu P, Cai J. 2018; Application of induced pluripotent stem cell transplants: autologous or allogeneic? Life Sci. 212:145–149. DOI: 10.1016/j.lfs.2018.09.057. PMID: 30290185. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85054181319&origin=inward.20. Tang D, Chen Y, He H, Huang J, Chen W, Peng W, Lu Q, Dai Y. 2016; Integrated analysis of mRNA, microRNA and protein in systemic lupus erythematosus-specific induced pluripotent stem cells from urine. BMC Genomics. 17:488. DOI: 10.1186/s12864-016-2809-9. PMID: 27402083. PMCID: PMC4940874. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84977676712&origin=inward.21. Liu C, Oikonomopoulos A, Sayed N, Wu JC. 2018; Modeling human diseases with induced pluripotent stem cells: from 2D to 3D and beyond. Development. 145:dev156166. DOI: 10.1242/dev.156166. PMID: 29519889. PMCID: PMC5868991. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85044319384&origin=inward.22. Brafman DA. 2013; Constructing stem cell microenvironments using bioengineering approaches. Physiol Genomics. 45:1123–1135. DOI: 10.1152/physiolgenomics.00099.2013. PMID: 24064536. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84888814075&origin=inward.23. Gattazzo F, Urciuolo A, Bonaldo P. 2014; Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim Biophys Acta. 1840:2506–2519. DOI: 10.1016/j.bbagen.2014.01.010. PMID: 24418517. PMCID: PMC4081568. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84903541437&origin=inward.24. Hew M, O'Connor K, Edel MJ, Lucas M. 2015; The possible future roles for iPSC-derived therapy for autoimmune diseases. J Clin Med. 4:1193–1206. DOI: 10.3390/jcm4061193. PMID: 26239553. PMCID: PMC4484994. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85020506503&origin=inward.25. Urowitz MB, Gladman DD, Tom BD, Ibañez D, Farewell VT. 2008; Changing patterns in mortality and disease outcomes for patients with systemic lupus erythematosus. J Rheumatol. 35:2152–2158. DOI: 10.3899/jrheum.080214. PMID: 18793004. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=56449114216&origin=inward.26. Chiong M, Wang ZV, Pedrozo Z, Cao DJ, Troncoso R, Ibacache M, Criollo A, Nemchenko A, Hill JA, Lavandero S. 2011; Cardiomyocyte death: mechanisms and translational implications. Cell Death Dis. 2:e244. DOI: 10.1038/cddis.2011.130. PMID: 22190003. PMCID: PMC3252742. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84862908305&origin=inward.27. Mohamed AAA, Hammam N, El Zohri MH, Gheita TA. 2019; Cardiac manifestations in systemic lupus erythematosus: clinical correlates of subclinical echocardiographic features. Biomed Res Int. 2019:2437105. DOI: 10.1155/2019/2437105. PMID: 30756081. PMCID: PMC6348873. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85060433144&origin=inward.28. Chen PY, Chang CH, Hsu CC, Liao YY, Chen KT. 2014; Systemic lupus erythematosus presenting with cardiac symptoms. Am J Emerg Med. 32:1117–1119. DOI: 10.1016/j.ajem.2014.06.036. PMID: 25082598. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84918543581&origin=inward.29. Guan X, Mack DL, Moreno CM, Strande JL, Mathieu J, Shi Y, Markert CD, Wang Z, Liu G, Lawlor MW, Moorefield EC, Jones TN, Fugate JA, Furth ME, Murry CE, Ruohola- Baker H, Zhang Y, Santana LF, Childers MK. 2014; Dystrophin- deficient cardiomyocytes derived from human urine: new biologic reagents for drug discovery. Stem Cell Res. 12:467–480. DOI: 10.1016/j.scr.2013.12.004. PMID: 24434629. PMCID: PMC3966181. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84892470978&origin=inward.30. Thatava T, Armstrong AS, De Lamo JG, Edukulla R, Khan YK, Sakuma T, Ohmine S, Sundsbak JL, Harris PC, Kudva YC, Ikeda Y. 2011; Successful disease-specific induced pluripotent stem cell generation from patients with kidney transplantation. Stem Cell Res Ther. 2:48. DOI: 10.1186/scrt89. PMID: 22142803. PMCID: PMC3340557. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84855683134&origin=inward.31. Son MY, Lee MO, Jeon H, Seol B, Kim JH, Chang JS, Cho YS. 2016; Generation and characterization of integration-free induced pluripotent stem cells from patients with autoimmune disease. Exp Mol Med. 48:e232. DOI: 10.1038/emm.2016.27. PMID: 27174201. PMCID: PMC4910148. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85009779039&origin=inward.32. Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D'Antoni ML, Debuque R, Chandran A, Wang L, Arora K, Rosenthal NA, Tallquist MD. 2016; Revisiting cardiac cellular composition. Circ Res. 118:400–409. DOI: 10.1161/CIRCRESAHA.115.307778. PMID: 26635390. PMCID: PMC4744092. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84958559113&origin=inward.33. Wu SM, Hochedlinger K. 2011; Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nat Cell Biol. 13:497–505. DOI: 10.1038/ncb0511-497. PMID: 21540845. PMCID: PMC3617981. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=79955572417&origin=inward.34. Behrens AN, Iacovino M, Lohr JL, Ren Y, Zierold C, Harvey RP, Kyba M, Garry DJ, Martin CM. 2013; Nkx2-5 mediates differential cardiac differentiation through interaction with Hoxa10. Stem Cells Dev. 22:2211–2220. DOI: 10.1089/scd.2012.0611. PMID: 23477547. PMCID: PMC3715789. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84880418959&origin=inward.35. Tanaka M, Chen Z, Bartunkova S, Yamasaki N, Izumo S. 1999; The cardiac homeobox gene Csx/Nkx2.5 lies genetically upstream of multiple genes essential for heart development. Development. 126:1269–1280. DOI: 10.1242/dev.126.6.1269. PMID: 10021345.36. Franceschini F, Cavazzana I. 2005; Anti-Ro/SSA and La/SSB antibodies. Autoimmunity. 38:55–63. DOI: 10.1080/08916930400022954. PMID: 15804706. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=17144427307&origin=inward.37. Catoggio LJ, Skinner RP, Smith G, Maddison PJ. 1984; Systemic lupus erythematosus in the elderly: clinical and serological characteristics. J Rheumatol. 11:175–181. PMID: 6610051. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0021247455&origin=inward.38. Praprotnik S, Bozic B, Kveder T, Rozman B. 1999; Fluctuation of anti-Ro/SS-A antibody levels in patients with systemic lupus erythematosus and Sjögren's syndrome: a prospective study. Clin Exp Rheumatol. 17:63–68. PMID: 10084034. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0032984338&origin=inward.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Is Stem Cell-Based Therapy Going on or Out for Cardiac Disease?
- Maturation of Stem Cell-Derived Cardiomyocytes: Foe in Translation Medicine
- Cardiac Regeneration with Human Pluripotent Stem Cell-Derived Cardiomyocytes
- From Bench to Market: Preparing Human Pluripotent Stem Cells Derived Cardiomyocytes for Various Applications
- Strategies for ensuring that regenerative cardiomyocytes function properly and in cooperation with the host myocardium