Korean J Gastroenterol.
2022 Jul;80(1):17-27. 10.4166/kjg.2022.025.
A Randomized, Double-blind, Active-controlled Exploratory Clinical Trial for the Evaluation of the Efficacy and Safety of Goodmorning S Granule® on Constipation
- Affiliations
-
- 1Division of Gastroenterology, Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- KMID: 2532163
- DOI: http://doi.org/10.4166/kjg.2022.025
Abstract
- Background/Aims
Constipation is a common gastrointestinal disease that reduces the quality of life and incurs considerable medical expenses. Bisacodyl and sodium docusate are generally used to treat constipation. This study assessed the effectiveness and safety of Goodmorning S Granule® (Hanpoong Pharm. Co., Ltd., Wanju, Korea) in functional constipation by a comparison with bisacodyl.
Methods
A 2-week randomized, double-blind, active-controlled exploratory clinical trial was conducted to compare the treatment (Goodmorning S Granule® ) with the control (bisacodyl). The efficacy was measured by the changes in transition, Bristol stool type, stomachache, clinical manifestation, defecation time after drug consumption, 36-item short-form survey (SF-36), and the results of improvement evaluation. The safety was evaluated by the incidence of adverse drug events and vital signs. Additional analyses were conducted by dividing the severity according to the proportion of Bristol Stool Scale types 1 and 2.
Results
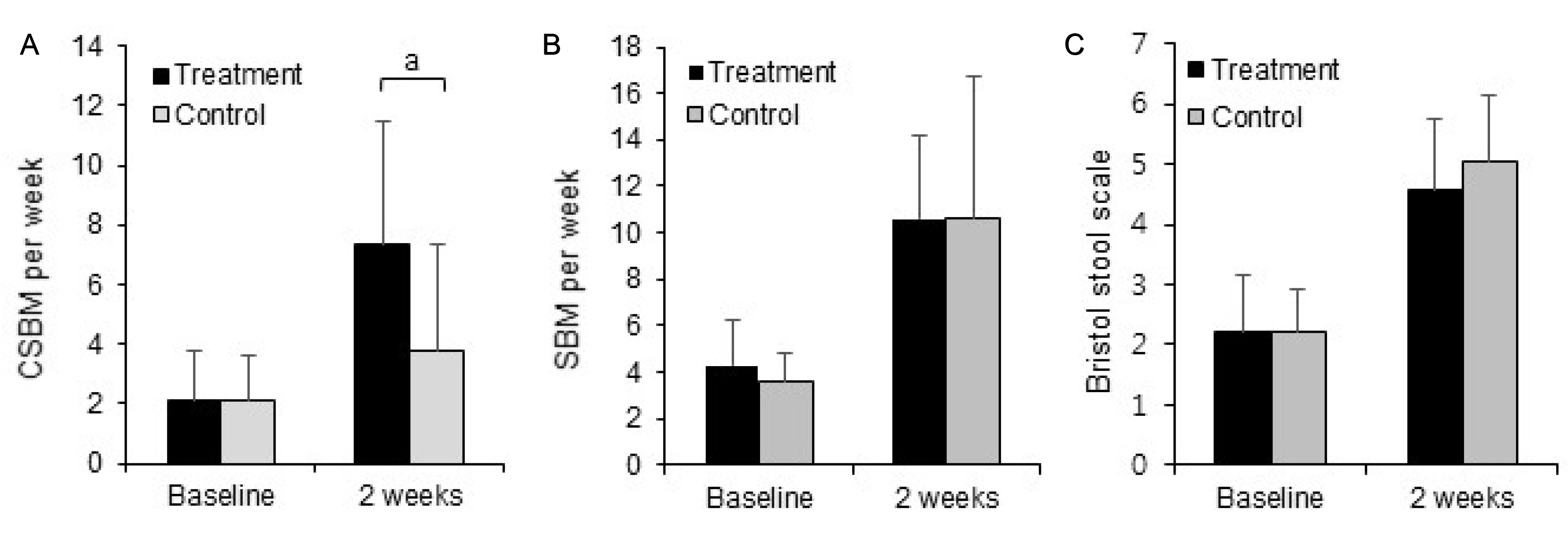
Subjects were randomized to the treatment (n=24) or control (n=26) groups. No significant differences were observed in demographics. After 2 weeks from the baseline, the changes in the complete spontaneous bowel movement (CSBM) were higher in the treatment (4.00±2.62) group than in the control group (1.40±2.34) (p<0.05). The treatment group exhibited significant improvement in the score on the SF-36 questionnaire. The clinical side effects, such as stomachache and borborygmus, were reduced in the moderate constipation patients in the treatment group, according to additional analyses.
Conclusions
Goodmorning S Granule® , a herbal medicine, was more effective in improving quality of life and CSBM per week and safer in the moderate constipation groups because of the reduced clinical side effects.
Keyword
Figure
Reference
-
1. Bharucha AE, Wald A. 2019; Chronic constipation. Mayo Clin Proc. 94:2340–2357. DOI: 10.1016/j.mayocp.2019.01.031. PMID: 31054770. PMCID: PMC6829047.2. Sood R, Ford AC. 2016; Diagnosis: Rome IV criteria for FGIDs - an improvement or more of the same? Nat Rev Gastroenterol Hepatol. 13:501–502. DOI: 10.1038/nrgastro.2016.110. PMID: 27407043.3. Wald A. 2016; Constipation: advances in diagnosis and treatment. JAMA. 315:185–191. DOI: 10.1001/jama.2015.16994. PMID: 26757467.4. Holtmann G, Talley NJ. 2015; Herbal medicines for the treatment of functional and inflammatory bowel disorders. Clin Gastroenterol Hepatol. 13:422–432. DOI: 10.1016/j.cgh.2014.03.014. PMID: 24674944.5. Fong HH. 2002; Integration of herbal medicine into modern medical practices: issues and prospects. Integr Cancer Ther. 1:287–293. DOI: 10.1177/153473540200100313. PMID: 14667286.6. Carmona F, Soares Pereira AM. 2013; Herbal medicines: old and new concepts, truths and misunderstandings. Rev Bras Farmacogn. 23:379–385. DOI: 10.1590/S0102-695X2013005000018.7. An SM, Kim HG, Choi EJ, et al. 2014; Screening for anti-inflammatory activities in extracts from Korean herb medicines. J Soc Cosmet Sci Korea. 40:95–108. DOI: 10.15230/scsk.2014.40.1.95.8. Gertsch J. 2011; Botanical drugs, synergy, and network pharmacology: forth and back to intelligent mixtures. Planta Med. 77:1086–1098. DOI: 10.1055/s-0030-1270904. PMID: 21412698.9. Rotblatt MD. 1999; Herbal medicine: a practical guide to safety and quality assurance. West J Med. 171:172–175. PMID: 10560292. PMCID: PMC1305803.10. Zhou X, Li CG, Chang D, Bensoussan A. 2019; Current status and major challenges to the safety and efficacy presented by Chinese herbal medicine. Medicines (Basel). 6:14. DOI: 10.3390/medicines6010014. PMID: 30669335. PMCID: PMC6473719.11. Williamson EM. 2001; Synergy and other interactions in phytomedicines. Phytomedicine. 8:401–409. DOI: 10.1078/0944-7113-00060. PMID: 11695885.12. Kim JH, Lee DJ, Yun SJ, Ahn SW, Kim YK. 2015; Building the database with herbal formulas based on the Korean medical classics. Herb Formula Sci. 23:209–224. DOI: 10.14374/HFS.2015.23.2.209.13. Kono T, Shimada M, Yamamoto M, et al. 2015; Complementary and synergistic therapeutic effects of compounds found in Kampo medicine: analysis of daikenchuto. Front Pharmacol. 6:159. DOI: 10.3389/fphar.2015.00159. PMID: 26300774. PMCID: PMC4523940.14. Iizuka N, Hamamoto Y. 2015; Constipation and herbal medicine. Front Pharmacol. 6:73. DOI: 10.3389/fphar.2015.00073. PMID: 25904866. PMCID: PMC4389350.15. Kon R, Ikarashi N, Nagoya C, et al. 2014; Rheinanthrone, a metabolite of sennoside A, triggers macrophage activation to decrease aquaporin-3 expression in the colon, causing the laxative effect of rhubarb extract. J Ethnopharmacol. 152:190–200. DOI: 10.1016/j.jep.2013.12.055. PMID: 24412547.16. Ogawa K, Tashima A, Okazaki K, Morinaga O. 2021; Evaluation of the amounts of sennosides A and B in rhubarb-containing Kampo medicines to create a ranking of Kampo medicines for appropriate selection of laxatives. J Anus Rectum Colon. 5:229–236. DOI: 10.23922/jarc.2020-102. PMID: 34395934. PMCID: PMC8321582.17. Bae SH. 2009; Medications for child with chronic constipation. Korean J Pediatr Gastroenterol Nutr. 12:S111–S117. DOI: 10.5223/kjpgn.2009.12.suppl1.s111.18. Slavin J. 2013; Fiber and prebiotics: mechanisms and health benefits. Nutrients. 5:1417–1435. DOI: 10.3390/nu5041417. PMID: 23609775. PMCID: PMC3705355.19. Zhu F, Xu S, Zhang Y, Chen F, Ji J, Xie G. 2016; Total glucosides of paeony promote intestinal motility in slow transit constipation rats through amelioration of interstitial cells of cajal. PLoS One. 11:e0160398. DOI: 10.1371/journal.pone.0160398. PMID: 27478893. PMCID: PMC4968804.20. Lim JH, Kim HS, Choi EJ, Shim CK, Park HJ. 2008; Effects of poncirus fructus on gastrointestinal motility in guinea pig: in vitro and in vivo study. Kor J Neurogastroenterol Motil. 14:7–17.21. Kim JH, Lee SK, Joo MC. 2016; Effects and safety of aqueous extract of poncirus fructus in spinal cord injury with neurogenic bowel. Evid Based Complement Alternat Med. 2016:7154616. DOI: 10.1155/2016/7154616. PMID: 27738444. PMCID: PMC5055929.22. Moon HJ, Lee SK, Noh SE, Joo MC. 2016; Effect of an aqueous extract of poncirus trifoliate (L.) Raf. in stroke patient with constipation. Journal of Korean Medicine Rehabilitation. 26:97–103. DOI: 10.18325/jkmr.2016.26.2.97.23. Kim SJ, Park KS. 2017; Pharmacotherapy in patients with chronic constipation. Korean J Gastroenterol. 70:64–71. DOI: 10.4166/kjg.2017.70.2.64. PMID: 28830131.24. Corsetti M, Landes S, Lange R. 2021; Bisacodyl: a review of pharmacology and clinical evidence to guide use in clinical practice in patients with constipation. Neurogastroenterol Motil. 33:e14123. DOI: 10.1111/nmo.14123. PMID: 33751780. PMCID: PMC8596401.25. Stimulant laxatives (bisacodyl, senna and sennosides, sodium picosulfate) available over-the-counter: new measures to support safe use. [Internet]. London: Medicines and Healthcare Products Regulatory Agency;2020. Aug. 18. cited 2022 Apr 1. Available from: https://www.gov.uk/drug-safety-update/stimulant-laxatives-bisacodyl-senna-and-sennosides-sodium-picosulfate-available-over-the-counter-new-measures-to-support-safe-use.26. Shin JE. 2017; Understanding the Rome IV: functional constipation and anorectal disorders. Korean J Med. 92:372–381. DOI: 10.3904/kjm.2017.92.4.372.27. You JS, Park JY, Chang KJ. 2010; Correlation among dietary habits score, life stress score and health-related quality of life (HRQL) score for female college students with functional constipation. Korean J Nutr. 43:620–627. DOI: 10.4163/kjn.2010.43.6.620.28. Schiller LR. 2001; Review article: the therapy of constipation. Aliment Pharmacol Ther. 15:749–763. DOI: 10.1046/j.1365-2036.2001.00982.x. PMID: 11380313.29. Siegel JD, Di Palma JA. 2005; Medical treatment of constipation. Clin Colon Rectal Surg. 18:76–80. DOI: 10.1055/s-2005-870887. PMID: 20011345. PMCID: PMC2780140.30. Mearin F, Ciriza C, Mínguez M, et al. 2016; Clinical practice guideline: irritable bowel syndrome with constipation and functional constipation in the adult. Rev Esp Enferm Dig. 108:332–363. DOI: 10.17235/reed.2016.4389/2016. PMID: 27230827.31. Lee B, Lee TH, Kim SE, et al. 2015; Conventional laxatives. Korean J Med. 88:1–8. DOI: 10.3904/kjm.2015.88.1.1. PMID: 21796580.32. Passmore AP, Wilson-Davies K, Stoker C, Scott ME. 1993; Chronic constipation in long stay elderly patients: a comparison of lactulose and a senna-fibre combination. BMJ. 307:769–771. DOI: 10.1136/bmj.307.6907.769. PMID: 8219947. PMCID: PMC1696423.33. Ramkumar D, Rao SS. 2005; Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol. 100:936–971. DOI: 10.1111/j.1572-0241.2005.40925.x. PMID: 15784043.34. Wald A, Scarpignato C, Kamm MA, et al. 2007; The burden of constipation on quality of life: results of a multinational survey. Aliment Pharmacol Ther. 26:227–236. DOI: 10.1111/j.1365-2036.2007.03376.x. PMID: 17593068.35. Belsey J, Greenfield S, Candy D, Geraint M. 2010; Systematic review: impact of constipation on quality of life in adults and children. Aliment Pharmacol Ther. 31:938–949. DOI: 10.1111/j.1365-2036.2010.04273.x. PMID: 20180788.36. Ruiz-López MC, Coss-Adame E. 2015; Quality of life in patients with different constipation subtypes based on the Rome III criteria. Rev Gastroenterol Mex. 80:13–20. DOI: 10.1016/j.rgmxen.2015.03.001. PMID: 25726441.37. Kwon HK, Do HJ, Kim HJ, et al. 2010; The impact of functional constipation on the quality of life in the elderly over 60 years. Korean J Fam Med. 31:35–43. DOI: 10.4082/kjfm.2010.31.1.35.38. Cho HS, Park JM, Lim CH, et al. 2011; Anxiety, depression and quality of life in patients with irritable bowel syndrome. Gut Liver. 5:29–36. DOI: 10.5009/gnl.2011.5.1.29. PMID: 21461069. PMCID: PMC3065090.39. Mason HJ, Serrano-Ikkos E, Kamm MA. 2002; Psychological state and quality of life in patients having behavioral treatment (biofeedback) for intractable constipation. Am J Gastroenterol. 97:3154–3159. DOI: 10.1111/j.1572-0241.2002.07113.x. PMID: 12492203.40. Glia A, Lindberg G. 1997; Quality of life in patients with different types of functional constipation. Scand J Gastroenterol. 32:1083–1089. DOI: 10.3109/00365529709002985. PMID: 9399387.41. Aziz I, Whitehead WE, Palsson OS, Törnblom H, Simrén M. 2020; An approach to the diagnosis and management of Rome IV functional disorders of chronic constipation. Expert Rev Gastroenterol Hepatol. 14:39–46. DOI: 10.1080/17474124.2020.1708718. PMID: 31893959.42. Yoon JH, Kim EH, Lee JY, Yoon SW. 2020; A literature review of management for opioid-induced constipation in cancer patients. J of Kor Traditional Oncology. 25:37–49.43. Basnayake C. 2018; Treatment of irritable bowel syndrome. Aust Prescr. 41:145–149. DOI: 10.18773/austprescr.2018.044. PMID: 30410210. PMCID: PMC6202292.44. Bharucha AE, Lacy BE. 2020; Mechanisms, evaluation, and management of chronic constipation. Gastroenterology. 158:1232–1249.e3. DOI: 10.1053/j.gastro.2019.12.034. PMID: 31945360. PMCID: PMC7573977.45. Kamm MA, Mueller-Lissner S, Wald A, Richter E, Swallow R, Gessner U. 2011; Oral bisacodyl is effective and well-tolerated in patients with chronic constipation. Clin Gastroenterol Hepatol. 9:577–583. DOI: 10.1016/j.cgh.2011.03.026. PMID: 21440672.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correspondence to editorial on “Safety and efficacy of HK-660S in patients with primary sclerosing cholangitis: A randomized double-blind phase 2a trial”
- Notice of Retraction: Pak CS, et al. A Phase III, Randomized, Double-Blind, Matched-Pairs, Active-Controlled Clinical Trial and Preclinical Animal Study to Compare the Durability, Efficacy and Safety between Polynucleotide Filler and Hyaluronic Acid Filler in the Correction of Crow's Feet: A New Concept of Regenerative Filler. J Korean Med Sci 2014; 29(Suppl 3): S201-S209
- A novel clinical trial for primary sclerosing cholangitis from Asia: All regional endeavors should improve global management of primary sclerosing cholangitis: Editorial on “Safety and efficacy of HK-660S in patients with primary sclerosing cholangitis: A randomized double-blind phase 2a trial”
- Efficacy and Safety of Low-Dose Gamma-Aminobutyric Acid From Unpolished Rice Germ as a Health Functional Food for Promoting Sleep: A Randomized, Double-Blind, Placebo-Controlled Trial
- Double-blind, Randomized, Multi-center, Comparative Clinical Trial of Sibutramine Mesilate with Sibutramine Hydrochloride for Evaluating Efficacy and Safety in Obese Patients