J Korean Med Sci.
2022 Jul;37(29):e227. 10.3346/jkms.2022.37.e227.
Objective Interpretation of the Rapid Urease Test for Helicobacter pylori Infection Using Colorimetry
- Affiliations
-
- 1Department of Gastroenterology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2School of Advanced Materials Science and Engineering, Sungkyunkwan University, Suwon, Korea
- 3Department of Microbiology, Hanyang University College of Medicine, Seoul, Korea
- KMID: 2532084
- DOI: http://doi.org/10.3346/jkms.2022.37.e227
Abstract
- Background
The rapid urease test (RUT) is a major diagnostic tool for detecting Helicobacter pylori infection. This study aimed to establish an objective method for measuring the color changes in the RUT kit to improve the test’s diagnostic accuracy.
Methods
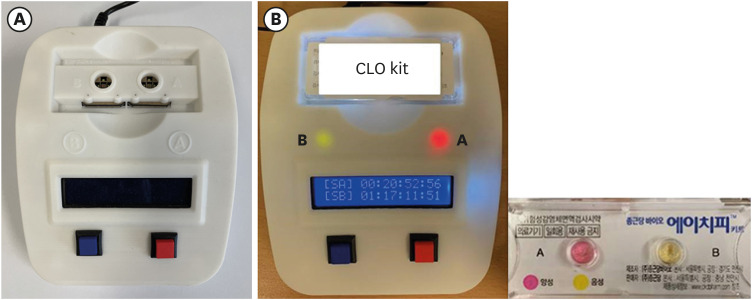
A UV-visible spectrophotometer was selected as the colorimeter; experiments were conducted in three stages to objectively identify the color changes in the RUT kit.
Results
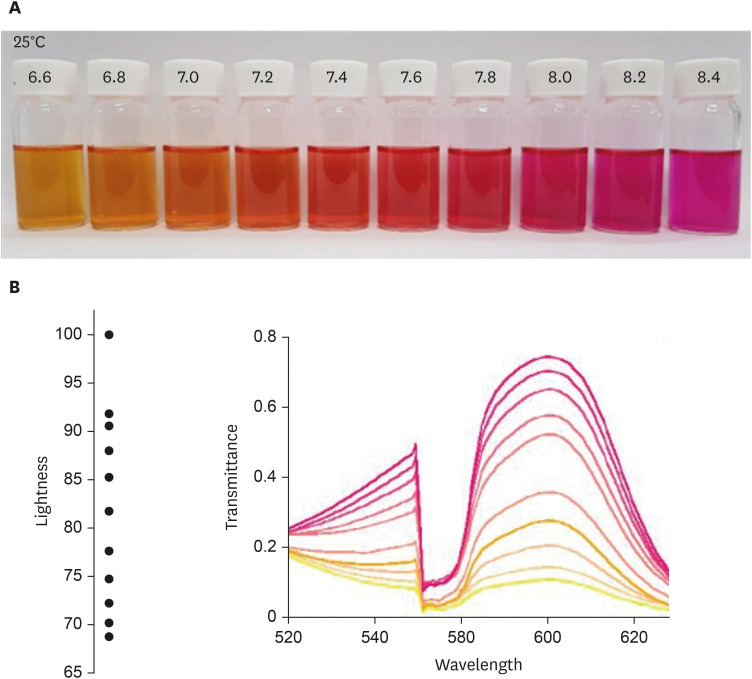
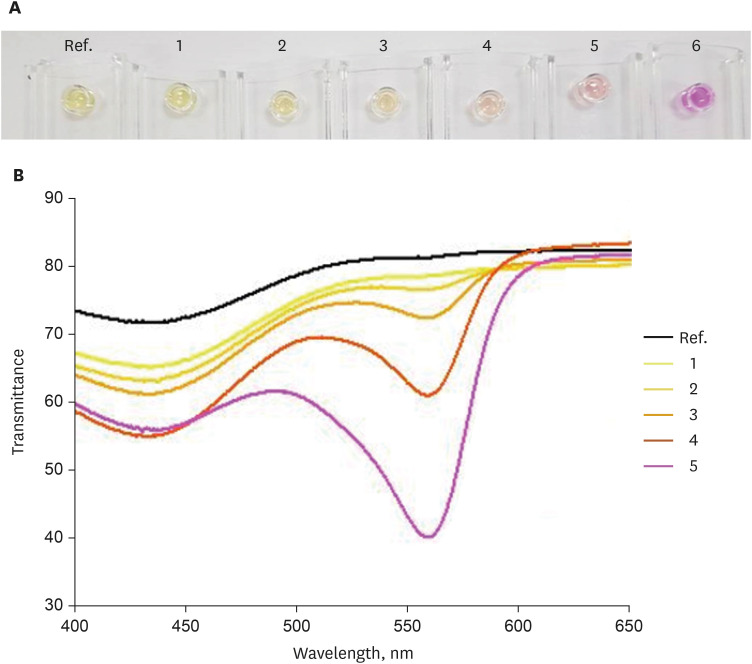
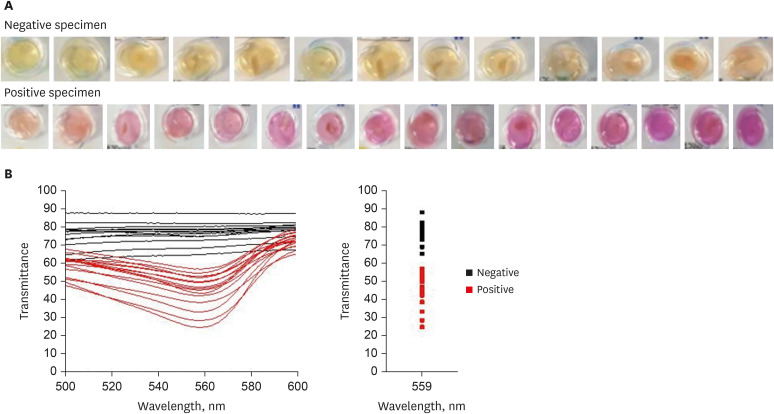
First, the urea broth solution showed an identifiable color change from yellow to red as the pH increased by 0.2. The largest transmittance difference detected using the UV-visible spectrophotometer was observed at a 590-nm wavelength. Second, the commercialized RUT kit also showed a gradual color change according to the pH change detected using the UV-visible spectrophotometer. Third, 13 cases of negative RUT results with a biopsy specimen and 16 of positive RUT results were collected. The transmittance detected using the UV-visible spectrophotometer showed a clear division between the positive and negative RUT groups; the largest difference was observed at a 559-nm wavelength. The lowest transmittance in the negative RUT group was 64, while the highest in the positive RUT group was 56, at the 559-nm wavelength. The UV-visible spectrophotometry reading showed a consistency of 92.7% compared with that of manual reading.
Conclusion
A transmittance of 60 at a 559-nm wavelength detected using UV-visible spectrophotometer can be used as a cutoff value for interpreting RUT results; this will help develop an automatic RUT kit reader with a high accuracy.
Figure
Reference
-
1. Kuipers EJ. Helicobacter pylori and the risk and management of associated diseases: gastritis, ulcer disease, atrophic gastritis and gastric cancer. Aliment Pharmacol Ther. 1997; 11(Suppl 1):71–88. PMID: 9146793.
Article2. Correa P, Houghton J. Carcinogenesis of Helicobacter pylori . Gastroenterology. 2007; 133(2):659–672. PMID: 17681184.3. Parsonnet J. Clinician-discoverers--Marshall, Warren, and H. pylori . N Engl J Med. 2005; 353(23):2421–2423. PMID: 16339090.4. Patel SK, Pratap CB, Jain AK, Gulati AK, Nath G. Diagnosis of Helicobacter pylori: what should be the gold standard? World J Gastroenterol. 2014; 20(36):12847–12859. PMID: 25278682.5. Lian DW, Xu YF, Deng QH, Lin XM, Huang B, Xian SX, et al. Effect of patchouli alcohol on macrophage mediated Helicobacter pylori digestion based on intracellular urease inhibition. Phytomedicine. 2019; 65:153097. PMID: 31568921.6. Puetz T, Vakil N, Phadnis S, Dunn B, Robinson J. The Pyloritek test and the CLO test: accuracy and incremental cost analysis. Am J Gastroenterol. 1997; 92(2):254–257. PMID: 9040201.7. Lee JH, Ahn JY, Choi KD, Jung HY, Kim JM, Baik GH, et al. Nationwide antibiotic resistance mapping of Helicobacter pylori in Korea: as prospective multicenter study. Helicobacter. 2019; 24(4):e12592. PMID: 31111572.8. Uotani T, Graham DY. Diagnosis of Helicobacter pylori using the rapid urease test. Ann Transl Med. 2015; 3(1):9. PMID: 25705641.9. Shirin H, Moss SF, Kancherla S, Kancherla K, Holt PR, Weinstein IB, et al. Non-steroidal anti-inflammatory drugs have bacteriostatic and bactericidal activity against Helicobacter pylori . J Gastroenterol Hepatol. 2006; 21(9):1388–1393. PMID: 16911681.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Diagnosis of Helicobacter pylori Infection
- Purification of the urease of helicobacter pylori and production of monoclonal antibody to the urease of helicobacter pylori
- An Alternative Method for a Rapid Urease Test Using Back-table Gastric Mucosal Biopsies from Gastrectomy Specimen for Making the Diagnosis of Helicobacter pylori Infection in Patients with Gastric Cancer
- Prevalence of Helicobacter pylori infection in patients of peptic ulcer among Korean people
- Serologic Diagnosis of Helicobacter pylori Gastritis in Children : Seroepidemiology of H. pylori in Normal School Children and Diagnostic Accuracy of IgG GAP Test in Children with Gastrointestinal Symptoms