Obstet Gynecol Sci.
2022 Jul;65(4):346-354. 10.5468/ogs.22017.
Multiple biomarkers are more accurate than a combination of carbohydrate antigen 125 and human epididymis protein 4 for ovarian cancer screening
- Affiliations
-
- 1BIOINFRA Life Science Inc., Seoul, Korea
- KMID: 2531843
- DOI: http://doi.org/10.5468/ogs.22017
Abstract
Objective
The objective of this study was to compare and evaluate the diagnostic value of serum carbohydrate antigen 125 (CA125) and/or human epididymis protein 4 (HE4) and a panel of novel multiple biomarkers in patients with ovarian tumors to identify more accurate and effective markers for screening ovarian cancer.
Methods
Candidate ovarian cancer biomarkers were selected based on a literature search. Dozens of candidate biomarkers were examined using 143 serum samples from patients with ovarian cancer and 157 healthy serum samples as noncancer controls. To select the optimal marker panel for an ovarian cancer classification model, a set of biomarker panels was created with the number of possible combinations of eight biomarkers. Using the set of biomarkers as an input variable, the optimal biomarker panel was selected by examining the performance of the biomarker panel set using the Random Forest algorithm as a non-linear classification method and a 10-fold cross-validation technique.
Results
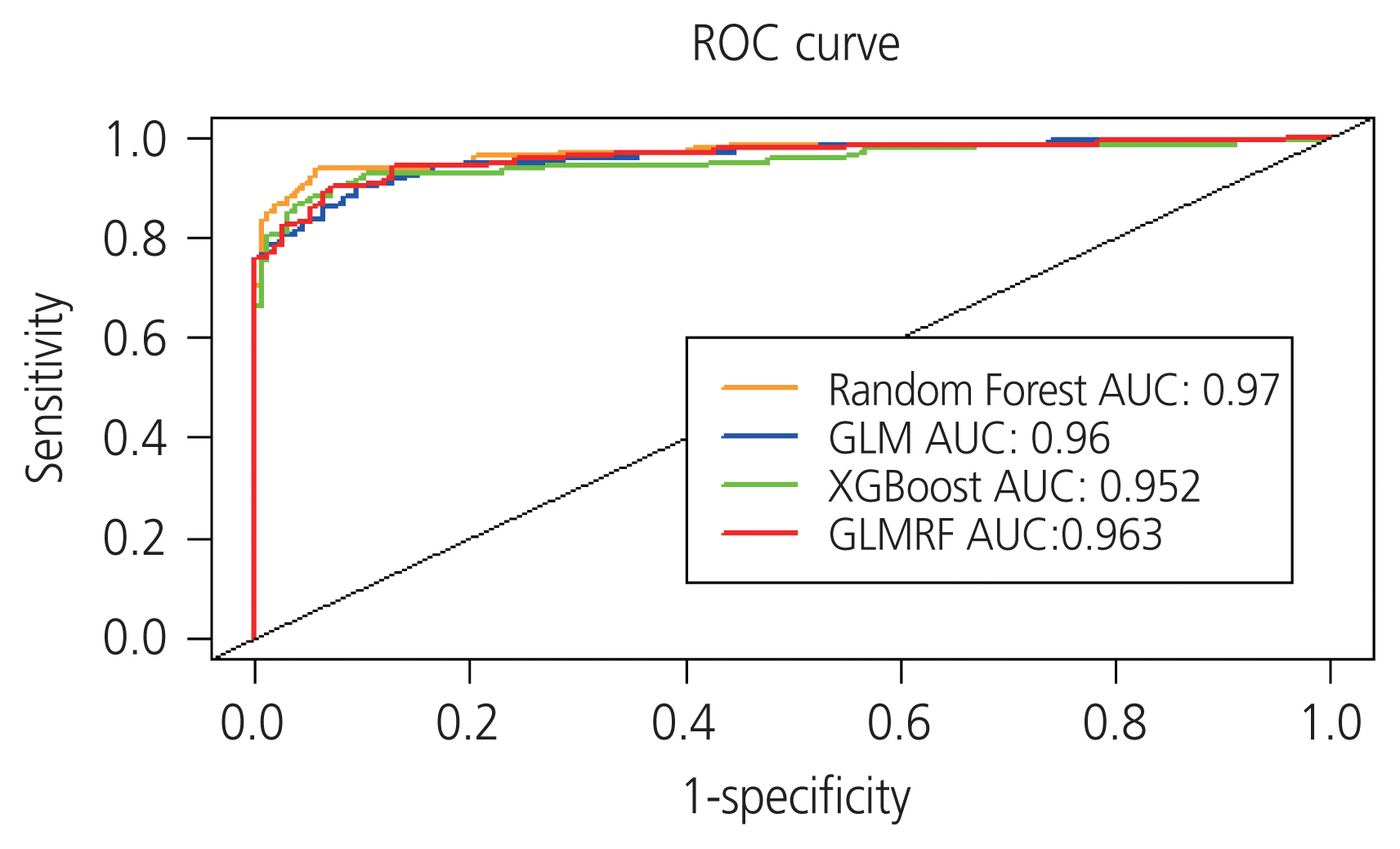
The final selected optimal combination of five biomarkers (CA125, HE4, cancer antigen 15-3, apolipoprotein [Apo] A1, and ApoA2) exhibited a sensitivity of 93.71% and specificity of 93.63% for ovarian cancer detection during validation.
Conclusion
Combining multiple biomarkers is a valid strategy for ovarian cancer diagnosis and can be used as a minimally invasive screening method for early ovarian cancer. A panel of five optimal biomarkers, including CA125 and HE4, was verified in this study. These can potentially be used as clinical biomarkers for early detection of ovarian cancer.
Keyword
Figure
Reference
-
References
1. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008; 58:71–96.
Article2. Ha HI, Chang HK, Park SJ, Lim J, Won YJ, Lim MC. The incidence and survival of cervical, ovarian, and endometrial cancer in Korea, 1999–2017: Korea Central Cancer Registry. Obstet Gynecol Sci. 2021; 64:444–53.
Article3. Heintz AP, Odicino F, Maisonneuve P, Quinn MA, Benedet JL, Creasman WT, et al. Carcinoma of the ovary. FIGO 26th annual report on the results of treatment in gynecological cancer. Int J Gynaecol Obstet. 2006; 95 (Suppl 1):S161–92.4. Brun JL, Fritel X, Levêque J. Clinical practice guidelines: presumed benign ovarian tumors--aims, methods, and organization. J Gynecol Obstet Biol Reprod (Paris). 2013; 42:710–4.5. Vaisbuch E, Dgani R, Ben-Arie A, Hagay Z. The role of laparoscopy in ovarian tumors of low malignant potential and early-stage ovarian cancer. Obstet Gynecol Surv. 2005; 60:326–30.
Article6. Marrugo-Ramírez J, Mir M, Samitier J. Blood-based cancer biomarkers in liquid biopsy: a promising non-invasive alternative to tissue biopsy. Int J Mol Sci. 2018; 19:2877.
Article7. Bates SE, Longo DL. Tumor markers: value and limitations in the management of cancer patients. Cancer Treat Rev. 1985; 12:163–207.
Article8. Holdenrieder S, Pagliaro L, Morgenstern D, Dayyani F. Clinically meaningful use of blood tumor markers in oncology. Biomed Res Int. 2016; 2016:9795269.
Article9. Urban N, McIntosh MW, Andersen M, Karlan BY. Ovarian cancer screening. Hematol Oncol Clin North Am. 2003; 17:989–1005.
Article10. Buamah P. Benign conditions associated with raised serum CA-125 concentration. J Surg Oncol. 2000; 75:264–5.
Article11. Hellström I, Raycraft J, Hayden-Ledbetter M, Ledbetter JA, Schummer M, McIntosh M, et al. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res. 2003; 63:3695–700.12. Ahmed AA, Abdou AM. Diagnostic accuracy of CA125 and HE4 in ovarian carcinoma patients and the effect of confounders on their serum levels. Curr Probl Cancer. 2019; 43:450–60.
Article13. Qing L, Xiaoling S, Yuqin Yang. Research progress of ROMA in early diagnosis of ovarian epithelial carcinoma. Adv Mod Biomed Sci. 2011; 11:4999–5000.14. Zhang Z. An in vitro diagnostic multivariate index assay (IVDMIA) for ovarian cancer: harvesting the power of multiple biomarkers. Rev Obstet Gynecol. 2012; 5:35–41.15. Lee HJ, Kim YT, Park PJ, Shin YS, Kang KN, Kim Y, et al. A novel detection method of non-small cell lung cancer using multiplexed bead-based serum biomarker profiling. J Thorac Cardiovasc Surg. 2012; 143:421–7.
Article16. Ahn HS, Shin YS, Park PJ, Kang KN, Kim Y, Lee HJ, et al. Serum biomarker panels for the diagnosis of gastric adenocarcinoma. Br J Cancer. 2012; 106:733–9.
Article17. Kim BK, Lee JW, Park PJ, Shin YS, Lee WY, Lee KA, et al. The multiplex bead array approach to identifying serum biomarkers associated with breast cancer. Breast Cancer Res. 2009; 11:R22.
Article18. Yoon HI, Kwon OR, Kang KN, Shin YS, Shin HS, Yeon EH, et al. Diagnostic value of combining tumor and inflammatory markers in lung cancer. J Cancer Prev. 2016; 21:187–93.
Article19. Fawzy A, Mohamed MR, Ali MA, Abd El-Magied MH, Helal AM. Tissue CA125 and HE4 gene expression levels offer superior accuracy in discriminating benign from malignant pelvic masses. Asian Pac J Cancer Prev. 2016; 17:323–33.
Article20. Zhuang H, Gao J, Hu Z, Liu J, Liu D, Lin B. Co-expression of Lewis Y antigen with human epididymis protein 4 in ovarian epithelial carcinoma. PLoS One. 2013; 8:e68994.
Article21. Drapkin R, von Horsten HH, Lin Y, Mok SC, Crum CP, Welch WR, et al. Human epididymis protein 4 (HE4) is a secreted glycoprotein that is overexpressed by serous and endometrioid ovarian carcinomas. Cancer Res. 2005; 65:2162–9.
Article22. Scambia G, Benedetti P, Baiocchi G, Perrone L, Greggi S, Di Roberto P, et al. CA 15-3 serum levels in ovarian cancer. Oncology. 1988; 45:263–7.
Article23. Lotzniker M, Pavesi F, Scarabelli M, Vadacca G, Franchi M, Moratti R. Tumour associated antigens CA 15.3 and CA 125 in ovarian cancer. Int J Biol Markers. 1991; 6:115–21.
Article24. Podzielinski I, Saunders BA, Kimbler KD, Branscum AJ, Fung ET, DePriest PD, et al. Apolipoprotein concentrations are elevated in malignant ovarian cyst fluids suggesting that lipoprotein metabolism is dysregulated in epithelial ovarian cancer. Cancer Invest. 2013; 31:258–72.
Article25. Zheng X, Chen S, Li L, Liu X, Liu X, Dai S, et al. Evaluation of HE4 and TTR for diagnosis of ovarian cancer: comparison with CA-125. J Gynecol Obstet Hum Reprod. 2018; 47:227–30.
Article26. Jin C, Yang M, Han X, Chu H, Zhang Y, Lu M, et al. Evaluation of the value of preoperative CYFRA21-1 in the diagnosis and prognosis of epithelial ovarian cancer in conjunction with CA125. J Ovarian Res. 2019; 12:114–21.
Article27. Sørensen SS, Mosgaard BJ. Combination of cancer antigen 125 and carcinoembryonic antigen can improve ovarian cancer diagnosis. Dan Med Bull. 2011; 58:A4331.28. Clarke-Pearson DL. Clinical practice. Screening for ovarian cancer. N Engl J Med. 2009; 361:170–7.29. Cho HY, Park SH, Park YH, Kim HB, Kang JB, Hong SH, et al. Comparison of HE4, CA125, and risk of ovarian malignancy algorithm in the prediction of ovarian cancer in Korean women. J Korean Med Sci. 2015; 30:1777–83.
Article30. Cui R, Wang Y, Li Y, Li Y. Clinical value of ROMA index in diagnosis of ovarian cancer: meta-analysis. Cancer Manag Res. 2019; 28:2545–51.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Human Epididymis Protein 4 as a Diagnostic Marker of Ovarian Cancer and Its Reference Interval in Korean Population
- Clinical efficacy of serum human epididymis protein 4 as a diagnostic biomarker of ovarian cancer: A pilot study
- The power of the Risk of Ovarian Malignancy Algorithm considering menopausal status: a comparison with CA 125 and HE4
- A clinical evaluation of CA 125 antigen values in patients of ovarian cancer
- Clinical Usefulness of Cancer Antigen (CA) 125, Human Epididymis 4, and CA72-4 Levels and Risk of Ovarian Malignancy Algorithm Values for Diagnosing Ovarian Tumors in Korean Patients With and Without Endometriosis