Decreased Serum Level of Sclerostin in Older Adults with Sarcopenia
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Inha University Hospital, Inha University College of Medicine, Incheon, Korea
- 2Division of Geriatrics, Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Asan Institute for Life Sciences, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 4Division of Endocrinology and Metabolism, Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2531392
- DOI: http://doi.org/10.3803/EnM.2022.1428
Abstract
- Background
Although muscles and bones interact with each other through various secretory factors, the role of sclerostin, an osteocyte-secreted factor, on muscle metabolism has not been well studied. We investigated the levels of serum sclerostin in Korean older adults with sarcopenia.
Methods
Blood samples were collected from 129 participants who underwent evaluation of muscle mass and function in an outpatient geriatric clinic of a teaching hospital. Sarcopenia and related parameters were determined using cutoff values for the Asian population. Serum sclerostin levels were measured using an enzyme-linked immunosorbent assay.
Results
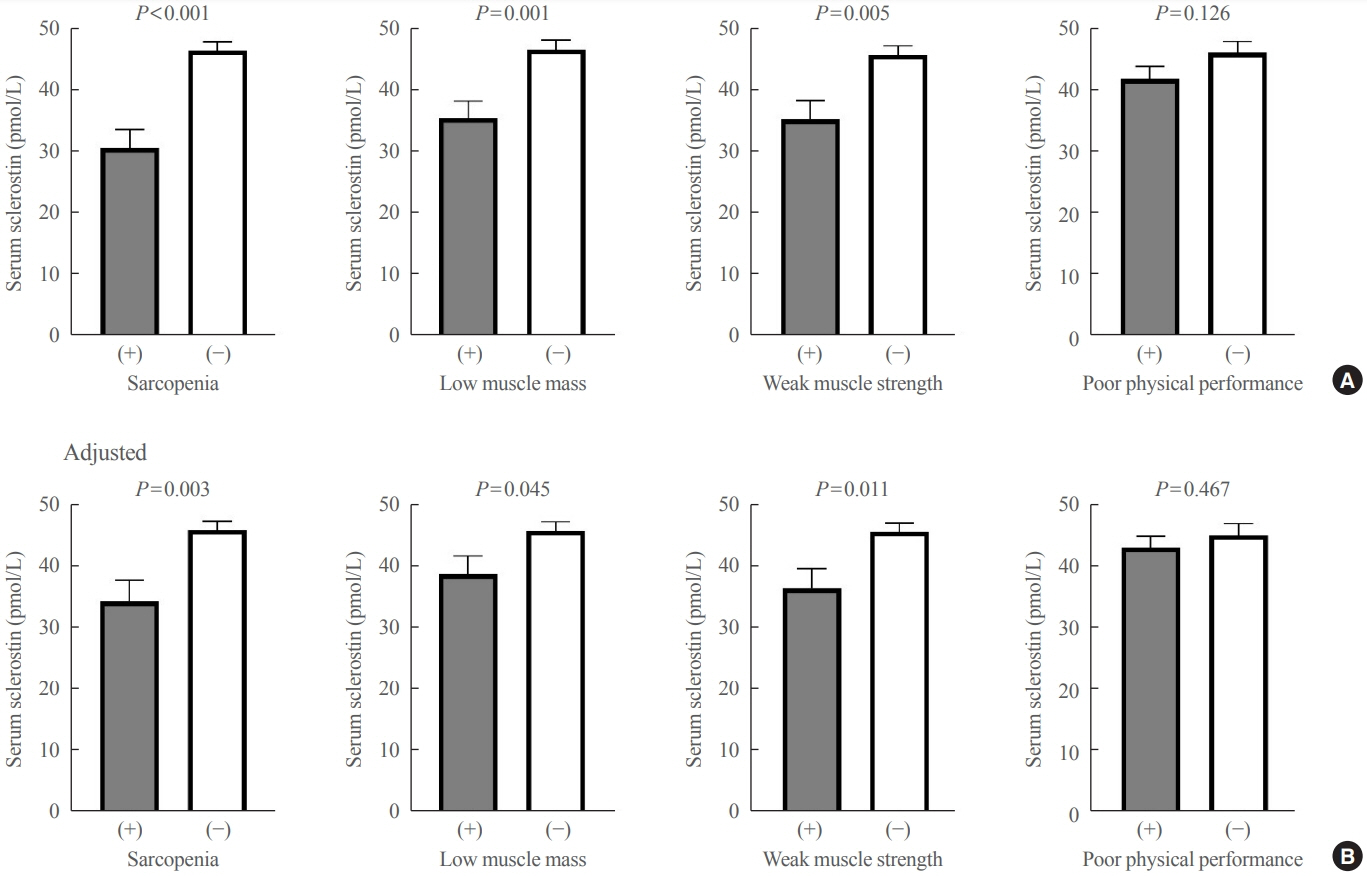
The mean age of the participants was 69.6 years, and 20 participants (15.5%) were classified as having sarcopenia. After adjusting for age, sex, and body mass index, serum sclerostin levels were significantly lower in participants with sarcopenia, low muscle mass, or weak muscle strength (P=0.003 to 0.045). Serum sclerostin levels were positively associated with skeletal muscle index and grip strength after adjusting for confounders (P=0.001 and P=0.003), whereas sarcopenic phenotype score showed a negative association (P=0.006). These increases in muscle mass and strength were also dose dependent as serum sclerostin levels increased (P for trends=0.003 and P for trends=0.015). Higher serum sclerostin levels were associated with lower odds ratio (ORs) for sarcopenia, low muscle mass, and weak muscle strength after adjusting for confounders (OR, 0.27 to 0.50; P<0.001 to 0.025).
Conclusion
Higher serum sclerostin levels were associated with a lower risk of sarcopenia, low muscle mass, and weak muscle strength in Korean older adults.
Keyword
Figure
Cited by 3 articles
-
Sclerostin as a Putative Myokine in Sarcopenia
Hyon-Seung Yi
Endocrinol Metab. 2022;37(3):430-431. doi: 10.3803/EnM.2022.303.Differences in Type 2 Fiber Composition in the Vastus Lateralis and Gluteus Maximus of Patients with Hip Fractures
Jingwen Tian, Minchul Song, Kyu Jeong Cho, Ho Yeop Lee, Sang Hyeon Ju, Jung Ryul Lim, Ha Thi Nga, Thi Linh Nguyen, Ji Sun Moon, Hyo Ju Jang, Jung-Mo Hwang, Hyon-Seung Yi
Endocrinol Metab. 2024;39(3):521-530. doi: 10.3803/EnM.2024.1935.Elevated Circulating Sclerostin Levels in Frail Older Adults: Implications beyond Bone Health
Ji Yeon Baek, Seong Hee Ahn, Il-Young Jang, Hee-Won Jung, Eunhye Ji, So Jeong Park, Yunju Jo, Eunju Lee, Dongryeol Ryu, Seongbin Hong, Beom-Jun Kim
Endocrinol Metab. 2025;40(1):73-81. doi: 10.3803/EnM.2024.2100.
Reference
-
1. Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997; 127(5 Suppl):990S–1S.
Article2. Dao T, Green AE, Kim YA, Bae SJ, Ha KT, Gariani K, et al. Sarcopenia and muscle aging: a brief overview. Endocrinol Metab (Seoul). 2020; 35:716–32.
Article3. Morley JE. Anorexia, sarcopenia, and aging. Nutrition. 2001; 17:660–3.
Article4. Beaudart C, Zaaria M, Pasleau F, Reginster JY, Bruyere O. Health outcomes of sarcopenia: a systematic review and meta-analysis. PLoS One. 2017; 12:e0169548.
Article5. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019; 48:16–31.
Article6. Yoo JI, Lee KH, Choi Y, Lee J, Park YG. Poor dietary protein intake in elderly population with sarcopenia and osteosarcopenia: a nationwide population-based study. J Bone Metab. 2020; 27:301–10.
Article7. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. 2019; 393:2636–46.
Article8. Osteoporosis prevention, diagnosis, and therapy. NIH Consens Statement. 2000; 17:1–45.9. Ahn SH, Park SM, Park SY, Yoo JI, Jung HS, Nho JH, et al. Osteoporosis and osteoporotic fracture fact sheet in Korea. J Bone Metab. 2020; 27:281–90.
Article10. Kirk B, Zanker J, Duque G. Osteosarcopenia: epidemiology, diagnosis, and treatment-facts and numbers. J Cachexia Sarcopenia Muscle. 2020; 11:609–18.
Article11. Tarantino U, Baldi J, Celi M, Rao C, Liuni FM, Iundusi R, et al. Osteoporosis and sarcopenia: the connections. Aging Clin Exp Res. 2013; 25 Suppl 1:S93–5.
Article12. Lu W, Xiao W, Xie W, Fu X, Pan L, Jin H, et al. The role of osteokines in sarcopenia: therapeutic directions and application prospects. Front Cell Dev Biol. 2021; 9:735374.
Article13. Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013; 19:179–92.
Article14. Clarke BL, Drake MT. Clinical utility of serum sclerostin measurements. Bonekey Rep. 2013; 2:361.
Article15. Modder UI, Hoey KA, Amin S, McCready LK, Achenbach SJ, Riggs BL, et al. Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J Bone Miner Res. 2011; 26:373–9.
Article16. Wang JS, Mazur CM, Wein MN. Sclerostin and osteocalcin: candidate bone-produced hormones. Front Endocrinol (Lausanne). 2021; 12:584147.
Article17. Oh JH, Song S, Rhee H, Lee SH, Kim DY, Choe JC, et al. Normal reference plots for the bioelectrical impedance vector in healthy Korean adults. J Korean Med Sci. 2019; 34:e198.
Article18. Jang IY, Jung HW, Lee CK, Yu SS, Lee YS, Lee E. Comparisons of predictive values of sarcopenia with different muscle mass indices in Korean rural older adults: a longitudinal analysis of the Aging Study of PyeongChang Rural Area. Clin Interv Aging. 2018; 13:91–9.
Article19. Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994; 49:M85–94.
Article20. Jung HW, Roh H, Cho Y, Jeong J, Shin YS, Lim JY, et al. Validation of a multi-sensor-based kiosk for Short Physical Performance Battery. J Am Geriatr Soc. 2019; 67:2605–9.
Article21. Jung HW, Roh HC, Kim SW, Kim S, Kim M, Won CW. Cross-comparisons of gait speeds by automatic sensors and a stopwatch to provide converting formula between measuring modalities. Ann Geriatr Med Res. 2019; 23:71–6.
Article22. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020; 21:300–7.
Article23. Jang IY, Lee E, Lee H, Park H, Kim S, Kim KI, et al. Characteristics of sarcopenia by European consensuses and a phenotype score. J Cachexia Sarcopenia Muscle. 2020; 11:497–504.
Article24. Daly RM, Rosengren BE, Alwis G, Ahlborg HG, Sernbo I, Karlsson MK. Gender specific age-related changes in bone density, muscle strength and functional performance in the elderly: a-10 year prospective population-based study. BMC Geriatr. 2013; 13:71.25. Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol (1985). 2000; 89:81–8.
Article26. Kirk B, Al Saedi A, Duque G. Osteosarcopenia: a case of geroscience. Aging Med (Milton). 2019; 2:147–56.
Article27. Sepulveda-Loyola W, Phu S, Bani Hassan E, Brennan-Olsen SL, Zanker J, Vogrin S, et al. The joint occurrence of osteoporosis and sarcopenia (osteosarcopenia): definitions and characteristics. J Am Med Dir Assoc. 2020; 21:220–5.
Article28. Greco EA, Pietschmann P, Migliaccio S. Osteoporosis and sarcopenia increase frailty syndrome in the elderly. Front Endocrinol (Lausanne). 2019; 10:255.
Article29. Yoo JI, Kim H, Ha YC, Kwon HB, Koo KH. Osteosarcopenia in patients with hip fracture is related with high mortality. J Korean Med Sci. 2018; 33:e27.
Article30. Kirk B, Feehan J, Lombardi G, Duque G. Muscle, bone, and fat crosstalk: the biological role of myokines, osteokines, and adipokines. Curr Osteoporos Rep. 2020; 18:388–400.
Article31. Avin KG, Bloomfield SA, Gross TS, Warden SJ. Biomechanical aspects of the muscle-bone interaction. Curr Osteoporos Rep. 2015; 13:1–8.
Article32. Brotto M, Bonewald L. Bone and muscle: interactions beyond mechanical. Bone. 2015; 80:109–14.
Article33. Karczewska-Kupczewska M, Stefanowicz M, Matulewicz N, Nikolajuk A, Straczkowski M. Wnt signaling genes in adipose tissue and skeletal muscle of humans with different degrees of insulin sensitivity. J Clin Endocrinol Metab. 2016; 101:3079–87.
Article34. Wood CL, Pajevic PD, Gooi JH. Osteocyte secreted factors inhibit skeletal muscle differentiation. Bone Rep. 2017; 6:74–80.
Article35. Phillips EG, Beggs LA, Ye F, Conover CF, Beck DT, Otzel DM, et al. Effects of pharmacologic sclerostin inhibition or testosterone administration on soleus muscle atrophy in rodents after spinal cord injury. PLoS One. 2018; 13:e0194440.
Article36. Huang J, Romero-Suarez S, Lara N, Mo C, Kaja S, Brotto L, et al. Crosstalk between MLO-Y4 osteocytes and C2C12 muscle cells is mediated by the Wnt/β-catenin pathway. JBMR Plus. 2017; 1:86–100.
Article37. Szulc P, Bertholon C, Borel O, Marchand F, Chapurlat R. Lower fracture risk in older men with higher sclerostin concentration: a prospective analysis from the MINOS study. J Bone Miner Res. 2013; 28:855–64.
Article38. Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008; 283:5866–75.39. Moustafa A, Sugiyama T, Prasad J, Zaman G, Gross TS, Lanyon LE, et al. Mechanical loading-related changes in osteocyte sclerostin expression in mice are more closely associated with the subsequent osteogenic response than the peak strains engendered. Osteoporos Int. 2012; 23:1225–34.
Article40. Lim Y, Kim CH, Lee SY, Kim H, Ahn SH, Lee SH, et al. Decreased plasma levels of sclerostin but not Dickkopf-1 are associated with an increased prevalence of osteoporotic fracture and lower bone mineral density in postmenopausal Korean women. Calcif Tissue Int. 2016; 99:350–9.
Article41. Yoshikawa T, Mori S, Santiesteban AJ, Sun TC, Hafstad E, Chen J, et al. The effects of muscle fatigue on bone strain. J Exp Biol. 1994; 188:217–33.
Article42. Kim JA, Roh E, Hong SH, Lee YB, Kim NH, Yoo HJ, et al. Association of serum sclerostin levels with low skeletal muscle mass: The Korean Sarcopenic Obesity Study (KSOS). Bone. 2019; 128:115053.
Article43. Magaro MS, Bertacchini J, Florio F, Zavatti M, Poti F, Cavani F, et al. Identification of sclerostin as a putative new myokine involved in the muscle-to-bone crosstalk. Biomedicines. 2021; 9:71.
Article44. Cosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016; 375:1532–43.
Article45. Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017; 377:1417–27.
Article46. Mockel L, Bartneck M, Mockel C. Risk of falls in postmenopausal women treated with romosozumab: preliminary indices from a meta-analysis of randomized, controlled trials. Osteoporos Sarcopenia. 2020; 6:20–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Elevated Circulating Sclerostin Levels in Frail Older Adults: Implications beyond Bone Health
- Romosozumab for the treatment of osteoporosis
- Circulating BMP-7 Level is Independent of Sarcopenia in Older Asian Adults
- Measurement of the Calf Muscle Circumference is Useful for Diagnosing Sarcopenia in Older Adults Requiring Long-Term Care
- Association of Diabetes Mellitus and Sarcopenia in Korean Adults