Cancer Res Treat.
2022 Jul;54(3):719-727. 10.4143/crt.2021.1019.
A Single-Arm, Prospective, Phase II Study of Cisplatin Plus Weekly Docetaxel as First-Line Therapy in Patients with Metastatic or Recurrent Salivary Gland Cancer
- Affiliations
-
- 1Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Division of Hematology-Oncology, Department of Medicine, Ewha Womans University School of Medicine, Seoul, Korea
- 3Department of Otorhinolaryngology-Head and Neck Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2531318
- DOI: http://doi.org/10.4143/crt.2021.1019
Abstract
- Purpose
Salivary gland cancers (SGCs) are relatively rare but comprise various histologic subtypes, which complicates design of prospective trials. Systemic chemotherapy plays a limited role in treatment of SGCs, but cisplatin and docetaxel showed efficacy in a previous preclinical study. Here, we conduct a prospective, phase II study to evaluate the efficacy and toxicities of cisplatin plus weekly docetaxel in patients with metastatic or recurrent SGC.
Materials and Methods
We included patients with histologically confirmed SGCs of the following subtypes: mucoepidermoid carcinoma, adenocarcinoma, ductal carcinoma, or adenoid cystic carcinoma. Patients had no prior systemic chemotherapy for metastatic or recurrent tumors and at least one measurable lesion. Patients were treated with docetaxel 35 mg/m2 (D1, 8) and cisplatin 70 mg/m2 (D1) every 21 days.
Results
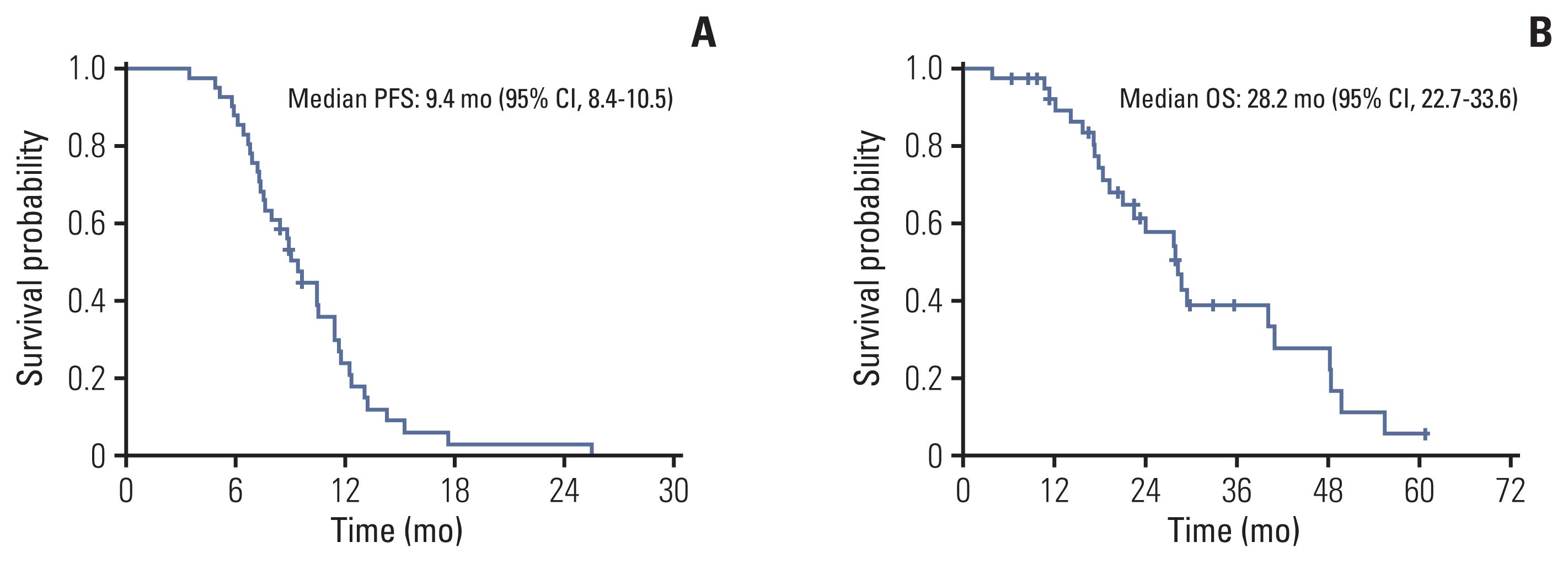
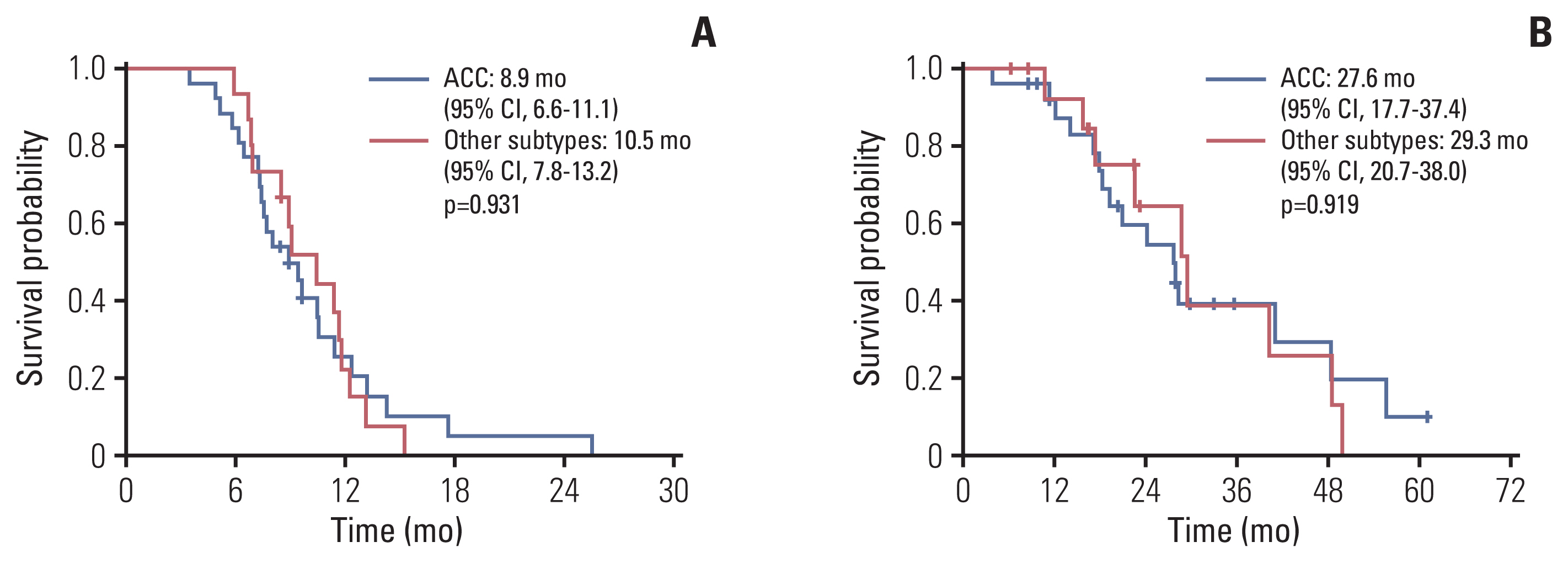
Forty-one patients were enrolled between April 2014 and October 2020. The median age was 58 years (range, 32 to 73 years). The most common histologic subtype was adenoid cystic carcinoma (63.4%), followed by ductal carcinoma (24.4%). The most common metastatic site was the lung (75.6%). The median treatment cycle was 5.5 (range, 3 to 8), and the objective response rate was 46.3%, with three complete responses. The median duration of response was 6.8 months (interquartile range, 4.0 to 10.2). The progression-free survival and overall survival were 9.4 months (95% confidence interval [CI], 8.4 to 10.5) and 28.2 months (95% CI, 22.7 to 33.6), respectively. There were no treatment-related deaths. The most common grade 3/4 adverse events were neutropenia (4.9%) and fatigue (4.9%).
Conclusion
Cisplatin plus weekly docetaxel is effective and tolerable with manageable toxicity as first-line therapy in patients with metastatic or recurrent SGC.
Figure
Reference
-
References
1. Laurie SA, Licitra L. Systemic therapy in the palliative management of advanced salivary gland cancers. J Clin Oncol. 2006; 24:2673–8.
Article2. Ciccolallo L, Licitra L, Cantu G, Gatta G; EUROCARE Working Group. Survival from salivary glands adenoid cystic carcinoma in European populations. Oral Oncol. 2009; 45:669–74.
Article3. van Weert S, Bloemena E, van der Waal I, de Bree R, Rietveld DH, Kuik JD, et al. Adenoid cystic carcinoma of the head and neck: a single-center analysis of 105 consecutive cases over a 30-year period. Oral Oncol. 2013; 49:824–9.
Article4. Rajasekaran K, Stubbs V, Chen J, Yalamanchi P, Cannady S, Brant J, et al. Mucoepidermoid carcinoma of the parotid gland: a National Cancer Database study. Am J Otolaryngol. 2018; 39:321–6.
Article5. Lassche G, van Boxtel W, Ligtenberg MJL, van Engen-van Grunsven AC, van Herpen CM. Advances and challenges in precision medicine in salivary gland cancer. Cancer Treat Rev. 2019; 80:101906.
Article6. Wang X, Luo Y, Li M, Yan H, Sun M, Fan T. Management of salivary gland carcinomas: a review. Oncotarget. 2017; 8:3946–56.7. Licitra L, Marchini S, Spinazze S, Rossi A, Rocca A, Grandi C, et al. Cisplatin in advanced salivary gland carcinoma: a phase II study of 25 patients. Cancer. 1991; 68:1874–7.
Article8. Hong MH, Kim CG, Koh YW, Choi EC, Kim J, Yoon SO, et al. Efficacy and safety of vinorelbine plus cisplatin chemotherapy for patients with recurrent and/or metastatic salivary gland cancer of the head and neck. Head Neck. 2018; 40:55–62.
Article9. Gilbert J, Li Y, Pinto HA, Jennings T, Kies MS, Silverman P, et al. Phase II trial of taxol in salivary gland malignancies (E1394): a trial of the Eastern Cooperative Oncology Group. Head Neck. 2006; 28:197–204.
Article10. Catimel G, Verweij J, Mattijssen V, Hanauske A, Piccart M, Wanders J, et al. Docetaxel (Taxotere): an active drug for the treatment of patients with advanced squamous cell carcinoma of the head and neck. EORTC Early Clinical Trials Group. Ann Oncol. 1994; 5:533–7.11. Raguse JD, Gath HJ, Bier J, Riess H, Oettle H. Docetaxel (Taxotere) in recurrent high grade mucoepidermoid carcinoma of the major salivary glands. Oral Oncol Extra. 2004; 40:5–7.
Article12. Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002; 346:92–8.
Article13. Gedlicka C, Formanek M, Selzer E, Burian M, Kornfehl J, Fiebiger W, et al. Phase II study with docetaxel and cisplatin in the treatment of recurrent and/or metastatic squamous cell carcinoma of the head and neck. Oncology. 2002; 63:145–50.
Article14. Korean Cancer Study Group (KCSG), Ji JH, Yun T, Kim SB, Kang JH, Park JC, et al. A prospective multicentre phase II study of cisplatin and weekly docetaxel as first-line treatment for recurrent or metastatic nasopharyngeal cancer (KCSG HN07-01). Eur J Cancer. 2012; 48:3198–204.
Article15. Laurie SA, Ho AL, Fury MG, Sherman E, Pfister DG. Systemic therapy in the management of metastatic or locally recurrent adenoid cystic carcinoma of the salivary glands: a systematic review. Lancet Oncol. 2011; 12:815–24.
Article16. Vered M, Braunstein E, Buchner A. Immunohistochemical study of epidermal growth factor receptor in adenoid cystic carcinoma of salivary gland origin. Head Neck. 2002; 24:632–6.
Article17. Yamada K, Iwai K, Okada Y, Mori M. Immunohistochemical expression of epidermal growth factor receptor in salivary gland tumours. Virchows Arch A Pathol Anat Histopathol. 1989; 415:523–31.
Article18. Hitre E, Budai B, Takacsi-Nagy Z, Rubovszky G, Toth E, Remenar E, et al. Cetuximab and platinum-based chemoradio- or chemotherapy of patients with epidermal growth factor receptor expressing adenoid cystic carcinoma: a phase II trial. Br J Cancer. 2013; 109:1117–22.
Article19. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010; 376:687–97.
Article20. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001; 344:783–92.
Article21. Egebjerg K, Harwood CD, Woller NC, Kristensen CA, Mau-Sorensen M. HER2 positivity in histological subtypes of salivary gland carcinoma: a systematic review and meta-analysis. Front Oncol. 2021; 11:693394.
Article22. Haddad R, Colevas AD, Krane JF, Cooper D, Glisson B, Amrein PC, et al. Herceptin in patients with advanced or metastatic salivary gland carcinomas: a phase II study. Oral Oncol. 2003; 39:724–7.
Article23. Agulnik M, Cohen EW, Cohen RB, Chen EX, Vokes EE, Hotte SJ, et al. Phase II study of lapatinib in recurrent or metastatic epidermal growth factor receptor and/or erbB2 expressing adenoid cystic carcinoma and non adenoid cystic carcinoma malignant tumors of the salivary glands. J Clin Oncol. 2007; 25:3978–84.24. Takahashi H, Tada Y, Saotome T, Akazawa K, Ojiri H, Fushimi C, et al. Phase II trial of trastuzumab and docetaxel in patients with human epidermal growth factor receptor 2-positive salivary duct carcinoma. J Clin Oncol. 2019; 37:125–34.
Article25. Kurzrock R, Bowles DW, Kang H, Meric-Bernstam F, Hainsworth J, Spigel DR, et al. Targeted therapy for advanced salivary gland carcinoma based on molecular profiling: results from MyPathway, a phase IIa multiple basket study. Ann Oncol. 2020; 31:412–21.
Article26. Li BT, Shen R, Offin M, Buonocore DJ, Myers ML, Venkatesh A, et al. Ado-trastuzumab emtansine in patients with HER2 amplified salivary gland cancers (SGCs): results from a phase II basket trial. J Clin Oncol. 2019; 37(15 Suppl):6001.
Article27. Bando H, Kinoshita I, Modi S, Tsurutani J, Bang YJ, Iwata H, et al. Trastuzumab deruxtecan (T-DXd) in patients with human epidermal growth factor receptor 2 (HER2)-expressing salivary duct carcinoma: subgroup analysis of two phase 1 studies. J Clin Oncol. 2021; 39(15 Suppl):6079.
Article28. Lim JJ, Kang S, Lee MR, Pai HK, Yoon HJ, Lee JI, et al. Expression of vascular endothelial growth factor in salivary gland carcinomas and its relation to p53, Ki-67 and prognosis. J Oral Pathol Med. 2003; 32:552–61.
Article29. Kim Y, Lee SJ, Lee JY, Lee SH, Sun JM, Park K, et al. Clinical trial of nintedanib in patients with recurrent or metastatic salivary gland cancer of the head and neck: a multicenter phase 2 study (Korean Cancer Study Group HN14-01). Cancer. 2017; 123:1958–64.
Article30. Chau NG, Hotte SJ, Chen EX, Chin SF, Turner S, Wang L, et al. A phase II study of sunitinib in recurrent and/or metastatic adenoid cystic carcinoma (ACC) of the salivary glands: current progress and challenges in evaluating molecularly targeted agents in ACC. Ann Oncol. 2012; 23:1562–70.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Multicenter Randomized Phase II Study of Docetaxel vs. Docetaxel Plus Cisplatin vs. Docetaxel Plus S-1 as Second-Line Chemotherapy in Metastatic Gastric Cancer Patients Who Had Progressed after Cisplatin Plus Either S-1 or Capecitabine
- Phase II Study of Docetaxel and Cisplatin as First-line Chemotherapy in Patients with Recurrent or Metastatic Gastric Cancer
- Combination chemotherapy with docetaxel and cisplatin as first-line treatment in advanced gastric cancer: is it a new effective chemotherapy?
- QL1604 plus paclitaxel-cisplatin/ carboplatin in patients with recurrent or metastatic cervical cancer: an open-label, single-arm, phase II trial
- Treatment of Advanced and Metastatic Squamous Non-small Cell Lung Cancer