Anat Cell Biol.
2022 Jun;55(2):205-216. 10.5115/acb.21.187.
Antioxidant, anti-inflammatory, and anti-fibrotic properties of olive leaf extract protect against L-arginine induced chronic pancreatitis in the adult male albino rat
- Affiliations
-
- 1Department of Human Anatomy and Embryology, Faculty of Medicine, Menoufia University, Menoufia, Egypt
- KMID: 2531207
- DOI: http://doi.org/10.5115/acb.21.187
Abstract
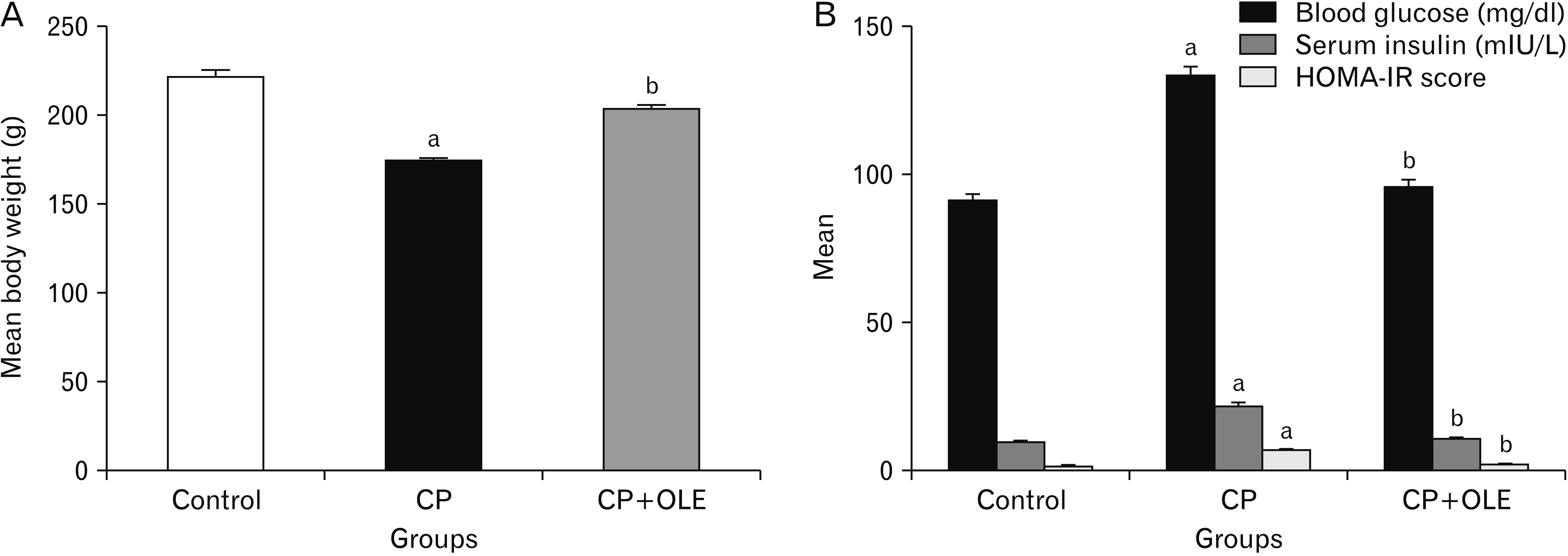
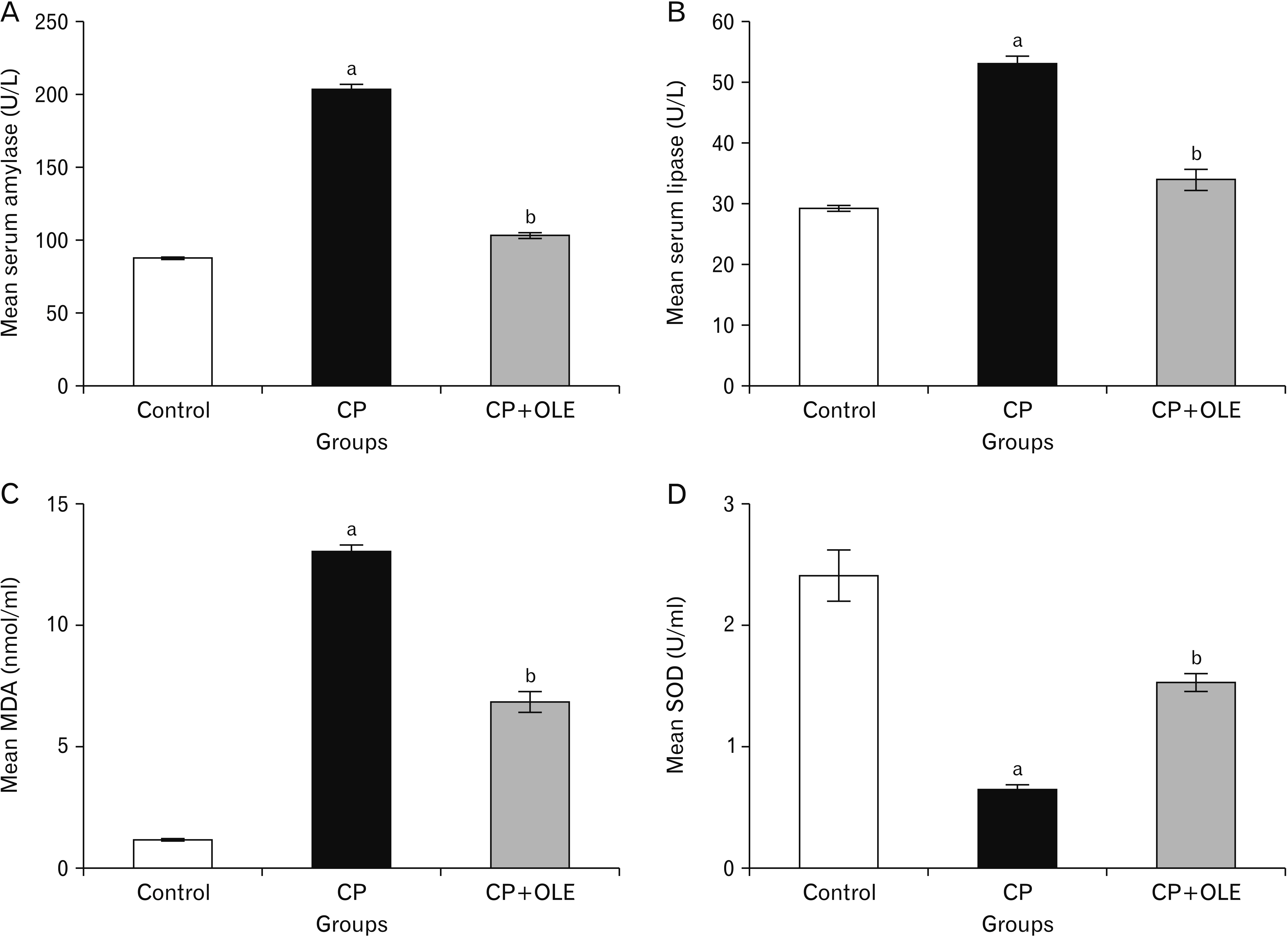
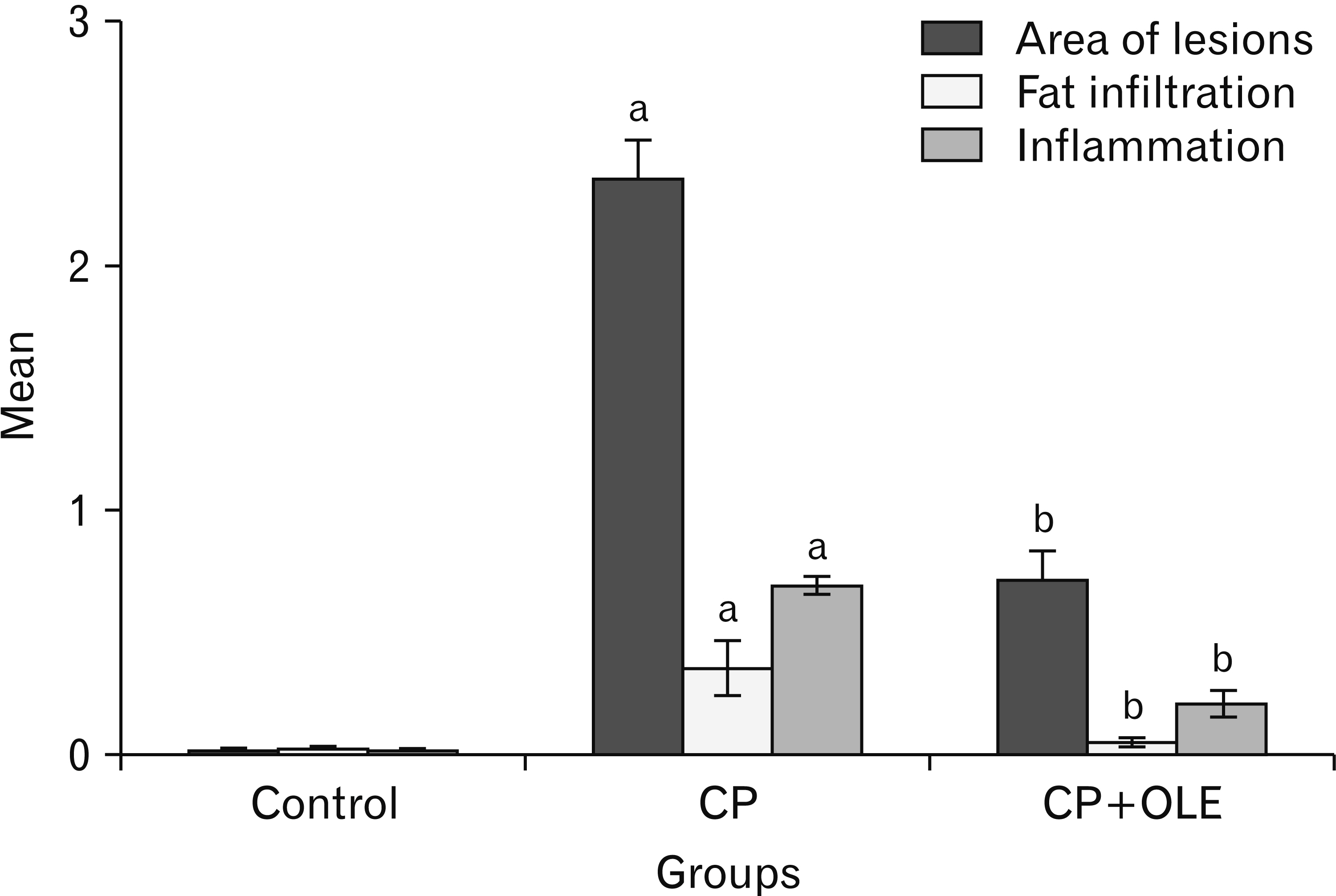
- Chronic pancreatitis (CP) is an inflammatory disease affects the pancreas with upcoming fibrosis and notable parenchymal destruction. CP poses a high risk for pancreatic carcinoma. The present study aimed to investigate, for the first time up to our knowledge, the effect of olive leaf extract on L-arginine induced CP with referral to some of its underlying mechanisms. Forty adult male albino rats were divided equally into four groups; control, olive leaf extract treated (200 mg/ kg orally once daily), CP group (300 mg L-arginine/100 g body weight intraperitoneally, once daily for 3 weeks then every 3 days for the subsequent 3 weeks), and CP treated with olive leaf extract group. At the end of the experiment, body weight, serum glucose, serum insulin, homeostatic model assessment of insulin resistance (HOMA-IR), serum amylase and lipase as well as tissue superoxide dismutase (SOD), and malondialdehyde (MDA) levels were assessed. Pancreatic tissues were subjected to histological and immuno-histochemical studies. The CP group revealed significant decrease in body weight and increase in serum glucose, serum insulin, HOMA-IR score, serum amylase, and serum lipase levels. Significant increase in MDA level and decrease in SOD level were detected. Marked degenerative changes and fibrosis were detected. Upregulation of alpha smooth muscle actin (α-SMA), transforming growth factor beta (TGF-β), caspase-3, and interleukin-6 (IL-6) immunoreactions were implicated in CP pathogenesis. Olive leaf extract alleviated all the examined parameters via itsantioxidant, anti-inflammatory, and anti-fibrotic properties. Olive leaf extract can protect against CP and restore pancreatic functions.
Keyword
Figure
Cited by 1 articles
-
Silymarin attenuates escitalopram (cipralex) induced pancreatic injury in adult male albino rats: a biochemical, histological, and immunohistochemical approach
Rasha Mamdouh Salama, Sara Gamal Tayel
Anat Cell Biol. 2023;56(1):122-136. doi: 10.5115/acb.22.204.
Reference
-
References
1. Whitcomb DC, Frulloni L, Garg P, Greer JB, Schneider A, Yadav D, Shimosegawa T. 2016; Chronic pancreatitis: an international draft consensus proposal for a new mechanistic definition. Pancreatology. 16:218–24. DOI: 10.1016/j.pan.2016.02.001. PMID: 26924663. PMCID: PMC6042966.
Article2. Hu FB. 2011; Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care. 34:1249–57. DOI: 10.2337/dc11-0442. PMID: 21617109. PMCID: PMC3114340.3. Otsuki M, Yamamoto M, Yamaguchi T. 2010; Animal models of chronic pancreatitis. Gastroenterol Res Pract. 2010:403295. DOI: 10.1155/2010/403295. PMID: 21197438. PMCID: PMC3010641.
Article4. Brock C, Nielsen LM, Lelic D, Drewes AM. 2013; Pathophysiology of chronic pancreatitis. World J Gastroenterol. 19:7231–40. DOI: 10.3748/wjg.v19.i42.7231. PMID: 24259953. PMCID: PMC3831204.
Article5. Melki G, Laham L, Karim G, Komal F, Kumar V, Barham S, Grossman M, Kuru S, Mohamed H, Garris R, Baddoura W. 2019; Chronic pancreatitis leading to pancreatogenic diabetes presenting in diabetic ketoacidosis: a rare entity. Gastroenterology Res. 12:208–10. DOI: 10.14740/gr1203. PMID: 31523331. PMCID: PMC6731042.
Article6. Zheng Z, Chen Y, Tan C, Ke N, Du B, Liu X. 2019; Risk of pancreatic cancer in patients undergoing surgery for chronic pancreatitis. BMC Surg. 19:83. DOI: 10.1186/s12893-019-0537-1. PMID: 31286902. PMCID: PMC6615265. PMID: 7706b5000bb245f9b3d232deaffec6b4.
Article7. Dong JY, Qin LQ, Zhang Z, Zhao Y, Wang J, Arigoni F, Zhang W. 2011; Effect of oral L-arginine supplementation on blood pressure: a meta-analysis of randomized, double-blind, placebo-controlled trials. Am Heart J. 162:959–65. DOI: 10.1016/j.ahj.2011.09.012. PMID: 22137067.
Article8. Meshram A, ivastava N Sr. 2015; Diverse potential and pharmacological studies of arginine. J Proteins Proteomics. 6:237–43.9. Malins LR, Cergol KM, Payne RJ. 2013; Peptide ligation-desulfurization chemistry at arginine. Chembiochem. 14:559–63. DOI: 10.1002/cbic.201300049. PMID: 23426906.
Article10. Sharma S, Rana SV, Nada R, Malhotra S, Rana S, Bhasin DK. 2017; Wheatgrass (Triticum Aestivum): an effective anti-oxidant in L-arginine induced chronic pancreatitis model of rat: a dose dependent study. Imp J Interdiscip Res. 3:245–51.11. Braganza JM, Lee SH, McCloy RF, McMahon MJ. 2011; Chronic pancreatitis. Lancet. 377:1184–97. DOI: 10.1016/S0140-6736(10)61852-1. PMID: 32798493.
Article12. Mayerle J, Hoffmeister A, Werner J, Witt H, Lerch MM, Mössner J. 2013; Chronic pancreatitis--definition, etiology, investigation and treatment. Dtsch Arztebl Int. 110:387–93. DOI: 10.3238/arztebl.2013.0387. PMID: 23826027. PMCID: PMC3698906.13. Apte MV, Pirola RC, Wilson JS. 2012; Pancreatic stellate cells: a starring role in normal and diseased pancreas. Front Physiol. 3:344. DOI: 10.3389/fphys.2012.00344. PMID: 22973234. PMCID: PMC3428781.
Article14. Tang D, Wang D, Yuan Z, Xue X, Zhang Y, An Y, Chen J, Tu M, Lu Z, Wei J, Jiang K, Miao Y. 2013; Persistent activation of pancreatic stellate cells creates a microenvironment favorable for the malignant behavior of pancreatic ductal adenocarcinoma. Int J Cancer. 132:993–1003. DOI: 10.1002/ijc.27715. PMID: 22777597.
Article15. Pothula SP, Xu Z, Goldstein D, Pirola RC, Wilson JS, Apte MV. 2016; Key role of pancreatic stellate cells in pancreatic cancer. Cancer Lett. 381:194–200. DOI: 10.1016/j.canlet.2015.10.035. PMID: 26571462.
Article16. Eidi A, Eidi M, Darzi R. 2009; Antidiabetic effect of Olea europaea L. in normal and diabetic rats. Phytother Res. 23:347–50. DOI: 10.1002/ptr.2629. PMID: 18844257.17. El SN, Karakaya S. 2009; Olive tree (Olea europaea) leaves: potential beneficial effects on human health. Nutr Rev. 67:632–8. DOI: 10.1111/j.1753-4887.2009.00248.x. PMID: 19906250.18. de Bock M, Derraik JG, Brennan CM, Biggs JB, Morgan PE, Hodgkinson SC, Hofman PL, Cutfield WS. 2013; Olive (Olea europaea L.) leaf polyphenols improve insulin sensitivity in middle-aged overweight men: a randomized, placebo-controlled, crossover trial. PLoS One. 8:e57622. DOI: 10.1371/journal.pone.0057622. PMID: 23516412. PMCID: PMC3596374. PMID: a0492c205ccf45dca7c6c494aa26b436.
Article19. Burja B, Kuret T, Janko T, Topalović D, Živković L, Mrak-Poljšak K, Spremo-Potparević B, Žigon P, Distler O, Čučnik S, Sodin-Semrl S, Lakota K, Frank-Bertoncelj M. 2019; Olive leaf extract attenuates inflammatory activation and DNA damage in human arterial endothelial cells. Front Cardiovasc Med. 6:56. DOI: 10.3389/fcvm.2019.00056. PMID: 31157238. PMCID: PMC6531989.
Article20. Boss A, Bishop KS, Marlow G, Barnett MP, Ferguson LR. 2016; Evidence to support the anti-cancer effect of olive leaf extract and future directions. Nutrients. 8:513. DOI: 10.3390/nu8080513. PMID: 27548217. PMCID: PMC4997426.
Article21. Al-Basher GI. 2018; Anti-fibrogentic and hepatoprotective potential of methanolic olive extract on cadmium induced toxicity in rats. Life Sci J. 15:1–11.22. Lin Z, Zheng LC, Zhang HJ, Tsang SW, Bian ZX. 2015; Anti-fibrotic effects of phenolic compounds on pancreatic stellate cells. BMC Complement Altern Med. 15:259. DOI: 10.1186/s12906-015-0789-y. PMID: 26223780. PMCID: PMC4520255.
Article23. Lins PG, Marina Piccoli Pugine S, Scatolini AM, de Melo MP. 2018; In vitro antioxidant activity of olive leaf extract (Olea europaea L.) and its protective effect on oxidative damage in human erythrocytes. Heliyon. 4:e00805. DOI: 10.1016/j.heliyon.2018.e00805. PMID: 30255162. PMCID: PMC6148714.24. Fredstrom SB, Jessurun J, Gallaher DD. 2009; Pancreatitis induced in rats by repetitive administration of L-arginine. Pancreas. 38:344–5. DOI: 10.1097/MPA.0b013e318184ff83. PMID: 19307932.
Article25. Al-Attar AM, Abu Zeid IM. 2013; Effect of tea (Camellia sinensis) and olive (Olea europaea L.) leaves extracts on male mice exposed to diazinon. Biomed Res Int. 2013:461415. DOI: 10.1155/2013/461415. PMID: 23691503. PMCID: PMC3652132.26. Al-Attar AM, Alsalmi FA. 2019; Effect of Olea europaea leaves extract on streptozotocin induced diabetes in male albino rats. Saudi J Biol Sci. 26:118–28. DOI: 10.1016/j.sjbs.2017.03.002. PMID: 30622415. PMCID: PMC6318816.
Article27. Judzewitsch RG, Pfeifer MA, Best JD, Beard JC, Halter JB, Porte D Jr. 1982; Chronic chlorpropamide therapy of noninsulin-dependent diabetes augments basal and stimulated insulin secretion by increasing islet sensitivity to glucose. J Clin Endocrinol Metab. 55:321–8. DOI: 10.1210/jcem-55-2-321. PMID: 7045153.
Article28. Foo AY, Bais R. 1998; Amylase measurement with 2-chloro-4-nitrophenyl maltotrioside as substrate. Clin Chim Acta. 272:137–47. DOI: 10.1016/S0009-8981(98)00009-6. PMID: 9641355.
Article29. Haffter D, Meyer N, Scholer A, Gyr K. 1983; Diagnostic value of the determination of serum amylase and serum lipase in suspected acute onset of acute or chronic pancreatitis. Schweiz Med Wochenschr. 113:184–8. German. PMID: 6188210.30. Kono Y. 1978; Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys. 186:189–95. DOI: 10.1016/0003-9861(78)90479-4. PMID: 24422.
Article31. Liu J, Xia Q, Zhang Q, Li H, Zhang J, Li A, Xiu R. 2009; Peroxisome proliferator-activated receptor-gamma ligands 15-deoxy-delta(12,14)-prostaglandin J2 and pioglitazone inhibit hydroxyl peroxide-induced TNF-alpha and lipopolysaccharide-induced CXC chemokine expression in neonatal rat cardiac myocytes. Shock. 32:317–24. DOI: 10.1097/SHK.0b013e31819c374c. PMID: 19174742.32. Koivukoski S. 2015. Assessing goblet cell metaplasia and expression of peptidase inhibitor 15 in mouse prostate tissue [Thesis]. Tampere University;Tampere:33. Christley RM. 2010; Power and error: increased risk of false positive results in underpowered studies. Open Epidemiol J. 3:16–9. DOI: 10.2174/1874297101003010016.
Article34. Valente R, Waldthaler A, Scandavini CM, Vujasinovic M, Del Chiaro M, Arnelo U, Löhr JM. 2020; Conservative treatment of chronic pancreatitis: a practical approach. Scand J Surg. 109:59–68. DOI: 10.1177/1457496920905559. PMID: 32192418.
Article35. Ramakrishnan P, Loh WM, Gopinath SCB, Bonam SR, Fareez IM, Mac Guad R, Sim MS, Wu YS. 2020; Selective phytochemicals targeting pancreatic stellate cells as new anti-fibrotic agents for chronic pancreatitis and pancreatic cancer. Acta Pharm Sin B. 10:399–413. DOI: 10.1016/j.apsb.2019.11.008. PMID: 32140388. PMCID: PMC7049637.
Article36. Khan FY, Matar I. 2007; Chylous ascites secondary to hyperlipidemic pancreatitis with normal serum amylase and lipase. World J Gastroenterol. 13:480–2. DOI: 10.3748/wjg.v13.i3.480. PMID: 17230625. PMCID: PMC4065911.
Article37. Dasgupta T, Hebbel RP, Kaul DK. 2006; Protective effect of arginine on oxidative stress in transgenic sickle mouse models. Free Radic Biol Med. 41:1771–80. DOI: 10.1016/j.freeradbiomed.2006.08.025. PMID: 17157180. PMCID: PMC1948977.
Article38. Zari TA, Al-Attar AM. 2011; Therapeutic effects of olive leaves extract on rats treated with a sublethal concentration of carbendazim. Eur Rev Med Pharmacol Sci. 15:413–26. PMID: 21608437.39. Bombardo M, Chen R, Malagola E, Saponara E, Hills AP, Graf R, Sonda S. 2018; Inhibition of class I histone deacetylases abrogates tumor growth factor β expression and development of fibrosis during chronic pancreatitis. Mol Pharmacol. 94:793–801. DOI: 10.1124/mol.117.110924. PMID: 29880639.
Article40. Geyikoglu F, Emir M, Colak S, Koc K, Turkez H, Bakir M, Hosseinigouzdagani M, Cerig S, Keles ON, Ozek NS. 2017; Effect of oleuropein against chemotherapy drug-induced histological changes, oxidative stress, and DNA damages in rat kidney injury. J Food Drug Anal. 25:447–59. DOI: 10.1016/j.jfda.2016.07.002. PMID: 28911689.
Article41. Bello M, Basilio-Antonio L, Fragoso-Vázquez J, Avalos-Soriano A, Correa-Basurto J. 2017; Molecular recognition between pancreatic lipase and natural and synthetic inhibitors. Int J Biol Macromol. 98:855–68. DOI: 10.1016/j.ijbiomac.2017.01.150. PMID: 28212930.
Article42. Schneider E, Schmid-Kotsas A, Zhao J, Weidenbach H, Schmid RM, Menke A, Adler G, Waltenberger J, Grünert A, Bachem MG. 2001; Identification of mediators stimulating proliferation and matrix synthesis of rat pancreatic stellate cells. Am J Physiol Cell Physiol. 281:C532–43. DOI: 10.1152/ajpcell.2001.281.2.C532. PMID: 11443052.
Article43. McCarroll JA, Naim S, Sharbeen G, Russia N, Lee J, Kavallaris M, Goldstein D, Phillips PA. 2014; Role of pancreatic stellate cells in chemoresistance in pancreatic cancer. Front Physiol. 5:141. DOI: 10.3389/fphys.2014.00141. PMID: 24782785. PMCID: PMC3988387.
Article44. Gong Z, Yuan Y, Lou K, Tu S, Zhai Z, Xu J. 2002; Mechanisms of Chinese herb emodin and somatostatin analogs on pancreatic regeneration in acute pancreatitis in rats. Pancreas. 25:154–60. DOI: 10.1097/00006676-200208000-00007. PMID: 12142738.
Article45. Mews P, Phillips P, Fahmy R, Korsten M, Pirola R, Wilson J, Apte M. 2002; Pancreatic stellate cells respond to inflammatory cytokines: potential role in chronic pancreatitis. Gut. 50:535–41. DOI: 10.1136/gut.50.4.535. PMID: 11889076. PMCID: PMC1773172.
Article46. Omary MB, Lugea A, Lowe AW, Pandol SJ. 2007; The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest. 117:50–9. DOI: 10.1172/JCI30082. PMID: 17200706. PMCID: PMC1716214.
Article47. Buchwalow I, Schnekenburger J, Tiemann K, Samoilova V, Bankfalvi A, Poremba C, Schleicher C, Neumann J, Boecker W. 2013; L-arginine-NO-cGMP signalling pathway in pancreatitis. Sci Rep. 3:1899. DOI: 10.1038/srep01899. PMID: 23712581. PMCID: PMC3664897.
Article48. Abdin AA, El-Hamid MA, El-Seoud SH, Balaha MF. 2010; Effect of pentoxifylline and/or alpha lipoic acid on experimentally induced acute pancreatitis. Eur J Pharmacol. 643:289–96. DOI: 10.1016/j.ejphar.2010.06.020. PMID: 20599924.
Article49. Al-Azzawie HF, Alhamdani MS. 2006; Hypoglycemic and antioxidant effect of oleuropein in alloxan-diabetic rabbits. Life Sci. 78:1371–7. DOI: 10.1016/j.lfs.2005.07.029. PMID: 16236331.
Article50. Abd El-Azim AO. 2014; Antioxidant effect of olive leaf extract on methotrexate-induced hepatic injury in rats. Canadian J Clin Nutr. 2:4–14. DOI: 10.14206/canad.j.clin.nutr.2014.01.02.
Article51. Al-Attar AM, Shawush NA. 2015; Influence of olive and rosemary leaves extracts on chemically induced liver cirrhosis in male rats. Saudi J Biol Sci. 22:157–63. DOI: 10.1016/j.sjbs.2014.08.005. PMID: 25737646. PMCID: PMC4336450.
Article52. Lobo V, Patil A, Phatak A, Chandra N. 2010; Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev. 4:118–26. DOI: 10.4103/0973-7847.70902. PMID: 22228951. PMCID: PMC3249911.
Article53. Cohen Z, Wilson J, Ritter L, McDonagh P. 2004; Caspase inhibition decreases both platelet phosphatidylserine exposure and aggregation: caspase inhibition of platelets. Thromb Res. 113:387–93. DOI: 10.1016/j.thromres.2004.03.020. PMID: 15226093.54. Mohammed HA, Okail HA, Ibrahim MA, Emam NM. 2018; Influences of olive leaf extract in the kidney of diabetic pregnant mice and their offspring. J Basic Appl Zool. 79:1–13. DOI: 10.1186/s41936-018-0024-8.
Article55. Pretnar-Oblak J, Sabovic M, Vidmar G, Zaletel M. 2007; Evaluation of L-arginine reactivity in comparison with flow-mediated dilatation and intima-media thickness. Ultrasound Med Biol. 33:1546–51. DOI: 10.1016/j.ultrasmedbio.2007.04.011. PMID: 17618037.
Article56. Badr A, Fouad D. 2016; Anti-apoptotic and anti-inflammatory effects of olive leaf extract against cisplatin-induced nephrotoxicity in male rats. Int J Pharmacol. 12:675–88. DOI: 10.3923/ijp.2016.675.688.
Article57. Rahman SH, Ammori BJ, Larvin M, McMahon MJ. 2003; Increased nitric oxide excretion in patients with severe acute pancreatitis: evidence of an endotoxin mediated inflammatory response? Gut. 52:270–4. DOI: 10.1136/gut.52.2.270. PMID: 12524412. PMCID: PMC1774972.
Article58. Eriksson JW. 2007; Metabolic stress in insulin's target cells leads to ROS accumulation - a hypothetical common pathway causing insulin resistance. FEBS Lett. 581:3734–42. DOI: 10.1016/j.febslet.2007.06.044. PMID: 17628546.
Article59. Hennig R, Kekis PB, Friess H, Adrian TE, Büchler MW. 2002; Pancreatic polypeptide in pancreatitis. Peptides. 23:331–8. DOI: 10.1016/S0196-9781(01)00605-2. PMID: 11825647.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-Inflammatory Effect of Phlorotannin on Chronic Nonbacterial Prostatitis in a Rat Model
- Antioxidant and anti-inflammatory effects and mechanism of Abeliophyllum distichum leaf extract in RAW264.7 macrophages
- Bisphosphonate’s effect on the tongue in adult male albino rats and the possible protective role of rutin: light and scanning electron microscopic study
- Anti-inflammatory, Anti-arthritic and Analgesic Effect of the Herbal Extract Made from Bacopa monnieriis, Cassia fistula and Phyllanthus polyphyllus
- Anti-nociceptive and Anti-inflammatory Effect of an Ethanol Extract Mixture of Vitis amurensis, Aralia cordata, and Glycyrrhizae radix