Diabetes Metab J.
2022 May;46(3):451-463. 10.4093/dmj.2021.0018.
Identification of Key Genes and Pathways in Peripheral Blood Mononuclear Cells of Type 1 Diabetes Mellitus by Integrated Bioinformatics Analysis
- Affiliations
-
- 1Department of Endocrinology, Jinling Hospital, Medical School of Nanjing University, Nanjing, China
- 2Department of Endocrinology, Jinling Hospital, Nanjing Medical University, Nanjing, China
- 3Department of Endocrinology, Translational Research Key Laboratory for Diabetes, Xinqiao Hospital, Army Medical University, Chongqing, China
- 4Department of Neurosurgery, The General Hospital of Chinese PLA Central Theater Command, Wuhan, China
- KMID: 2530184
- DOI: http://doi.org/10.4093/dmj.2021.0018
Abstract
- Background
The onset and progression of type 1 diabetes mellitus (T1DM) is closely related to autoimmunity. Effective monitoring of the immune system and developing targeted therapies are frontier fields in T1DM treatment. Currently, the most available tissue that reflects the immune system is peripheral blood mononuclear cells (PBMCs). Thus, the aim of this study was to identify key PBMC biomarkers of T1DM.
Methods
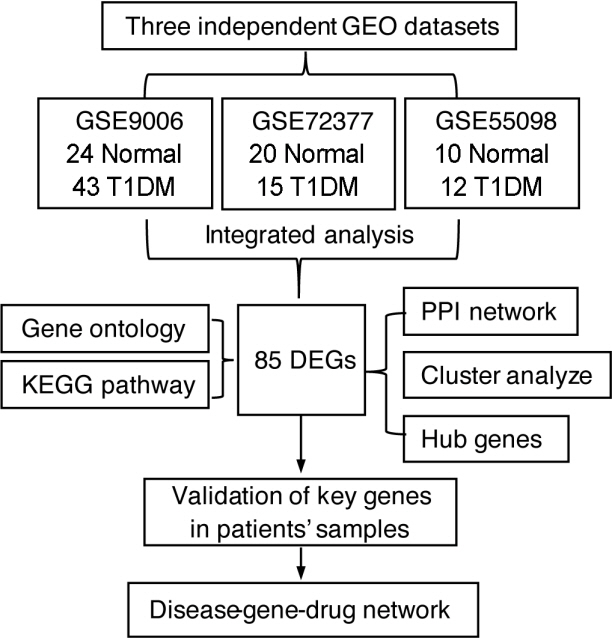
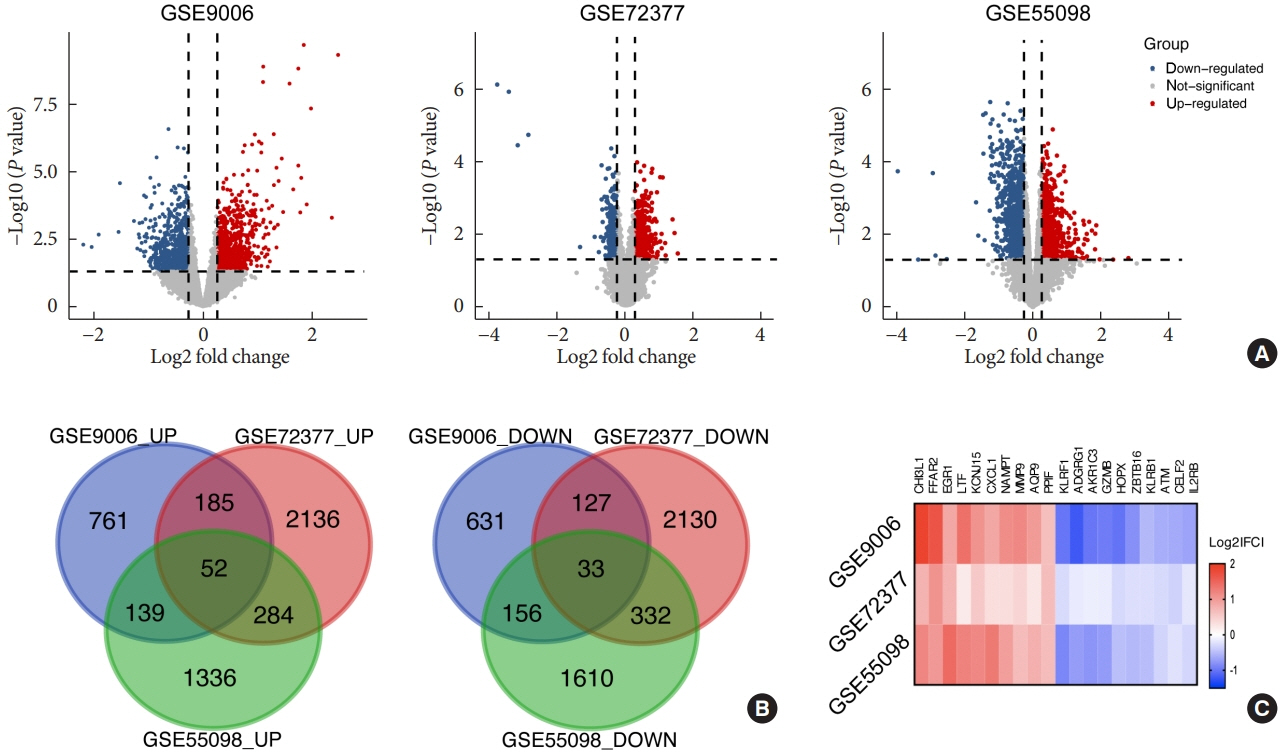
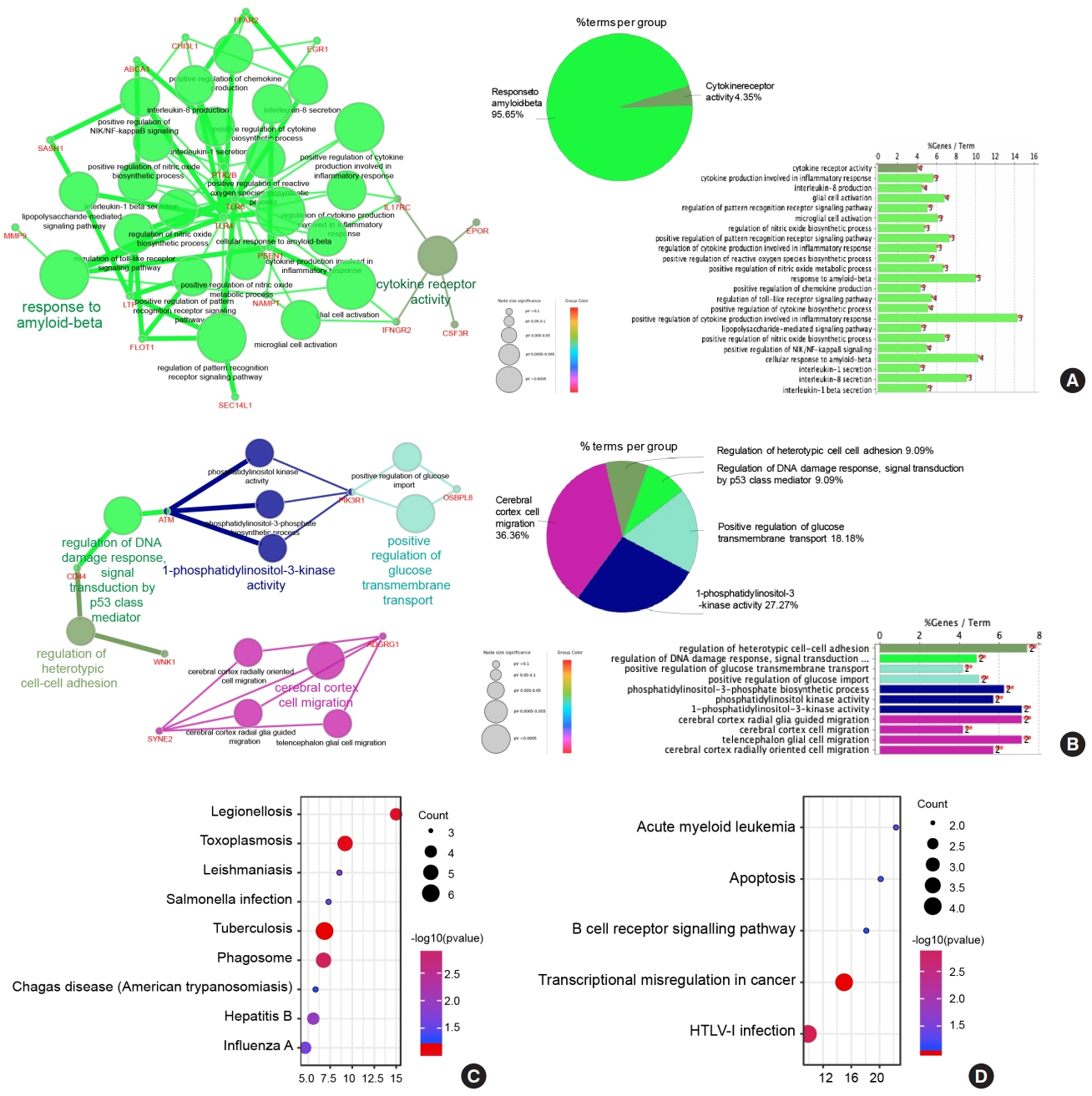
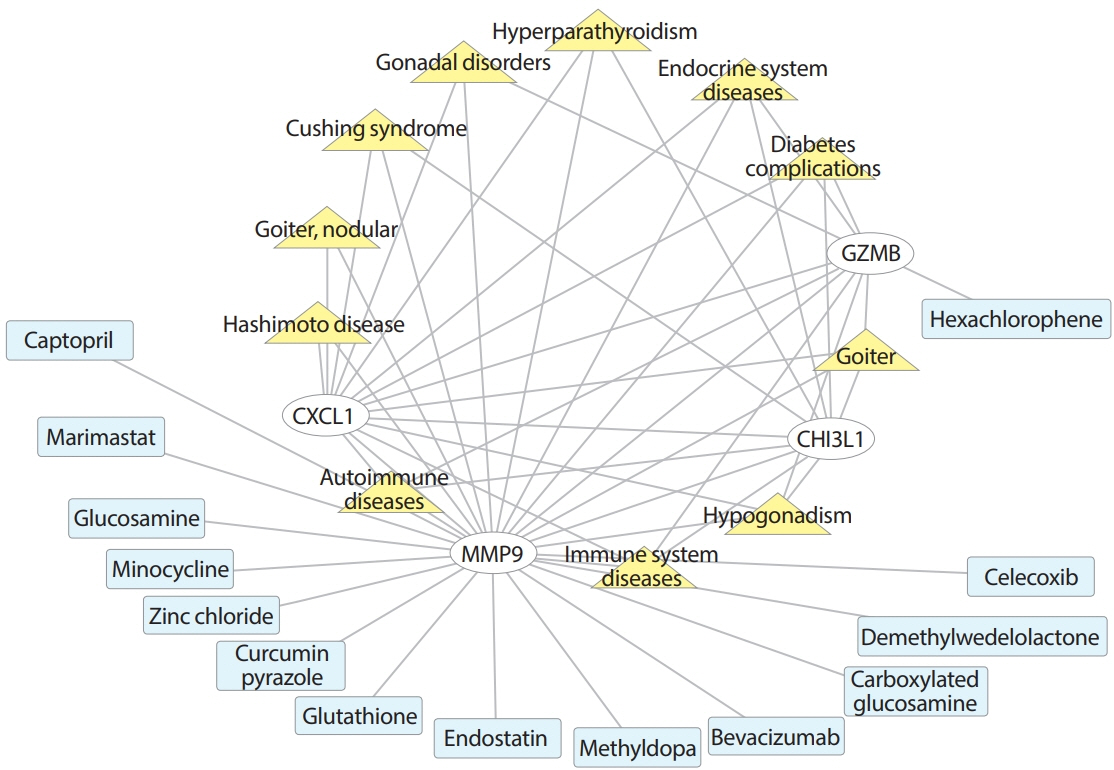
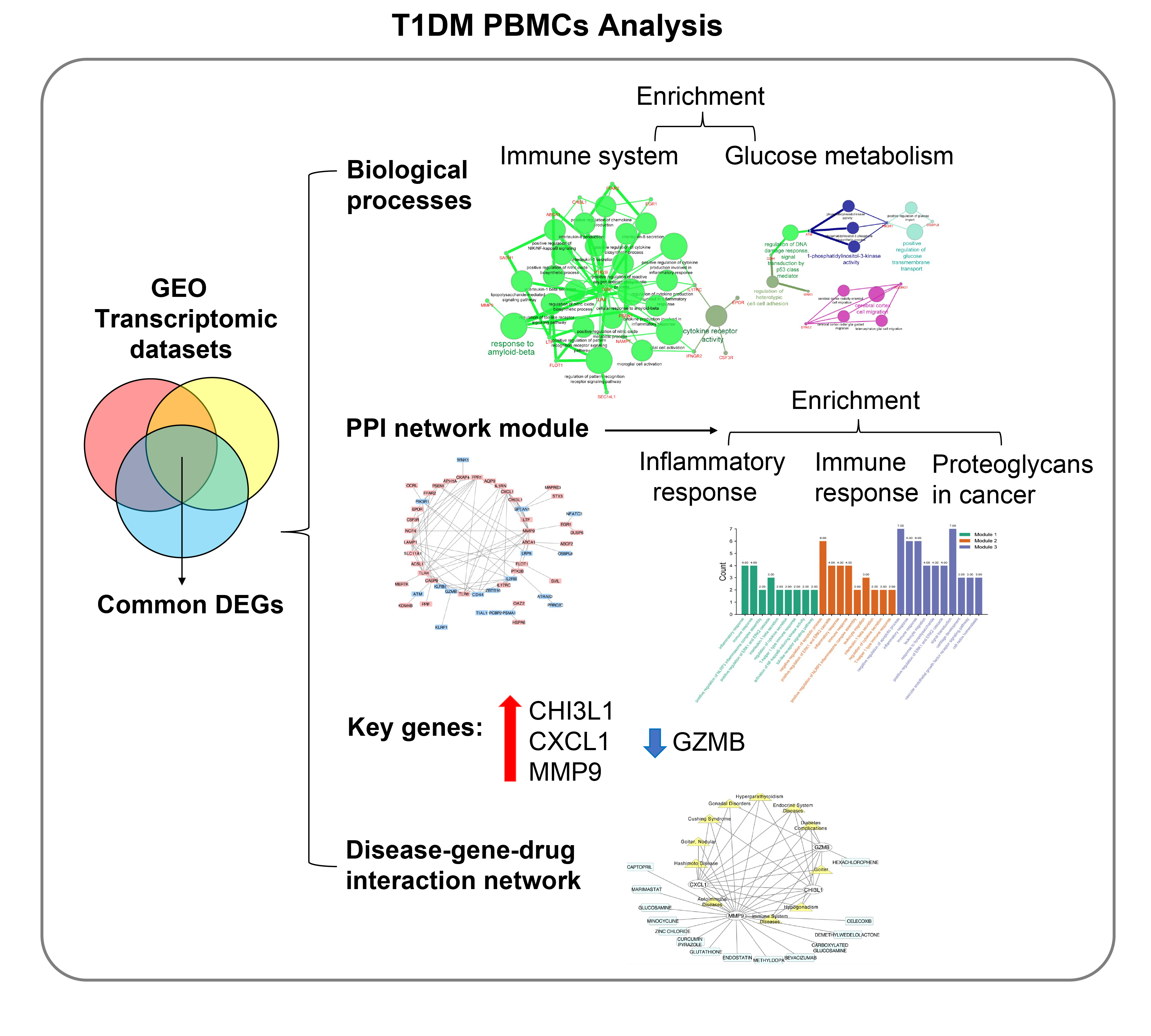
Common differentially expressed genes (DEGs) were screened from the Gene Expression Omnibus (GEO) datasets GSE9006, GSE72377, and GSE55098, and PBMC mRNA expression in T1DM patients was compared with that in healthy participants by GEO2R. Gene Ontology, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway and protein-protein interaction (PPI) network analyses of DEGs were performed using the Cytoscape, DAVID, and STRING databases. The vital hub genes were validated by reverse transcription-polymerase chain reaction using clinical samples. The disease-gene-drug interaction network was built using the Comparative Toxicogenomics Database (CTD) and Drug Gene Interaction Database (DGIdb).
Results
We found that various biological functions or pathways related to the immune system and glucose metabolism changed in PBMCs from T1DM patients. In the PPI network, the DEGs of module 1 were significantly enriched in processes including inflammatory and immune responses and in pathways of proteoglycans in cancer. Moreover, we focused on four vital hub genes, namely, chitinase-3-like protein 1 (CHI3L1), C-X-C motif chemokine ligand 1 (CXCL1), matrix metallopeptidase 9 (MMP9), and granzyme B (GZMB), and confirmed them in clinical PBMC samples. Furthermore, the disease-gene-drug interaction network revealed the potential of key genes as reference markers in T1DM.
Conclusion
These results provide new insight into T1DM pathogenesis and novel biomarkers that could be widely representative reference indicators or potential therapeutic targets for clinical applications.
Keyword
Figure
Reference
-
1. Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010; 464:1293–300.
Article2. Chiang JL, Kirkman MS, Laffel LM, Peters AL; Type 1 Diabetes Sourcebook Authors. Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care. 2014; 37:2034–54.
Article3. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018; 392:1789–858.4. McKnight JA, Wild SH, Lamb MJ, Cooper MN, Jones TW, Davis EA, et al. Glycaemic control of type 1 diabetes in clinical practice early in the 21st century: an international comparison. Diabet Med. 2015; 32:1036–50.5. Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002-2012. N Engl J Med. 2017; 376:1419–29.
Article6. Herold KC, Vignali DA, Cooke A, Bluestone JA. Type 1 diabetes: translating mechanistic observations into effective clinical outcomes. Nat Rev Immunol. 2013; 13:243–56.
Article7. Bulek AM, Cole DK, Skowera A, Dolton G, Gras S, Madura F, et al. Structural basis for the killing of human beta cells by CD8(+) T cells in type 1 diabetes. Nat Immunol. 2012; 13:283–9.
Article8. Ferreira RC, Guo H, Coulson RM, Smyth DJ, Pekalski ML, Burren OS, et al. A type I interferon transcriptional signature precedes autoimmunity in children genetically at risk for type 1 diabetes. Diabetes. 2014; 63:2538–50.
Article9. Beyan H, Drexhage RC, van der Heul Nieuwenhuijsen L, de Wit H, Padmos RC, Schloot NC, et al. Monocyte gene-expression profiles associated with childhood-onset type 1 diabetes and disease risk: a study of identical twins. Diabetes. 2010; 59:1751–5.
Article10. Kaizer EC, Glaser CL, Chaussabel D, Banchereau J, Pascual V, White PC. Gene expression in peripheral blood mononuclear cells from children with diabetes. J Clin Endocrinol Metab. 2007; 92:3705–11.
Article11. Yang M, Ye L, Wang B, Gao J, Liu R, Hong J, et al. Decreased miR-146 expression in peripheral blood mononuclear cells is correlated with ongoing islet autoimmunity in type 1 diabetes patients 1miR-146. J Diabetes. 2015; 7:158–65.
Article12. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2007; 30 Suppl 1:S42–7.13. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011; 34 Suppl 1:S62–9.14. Bindea G, Mlecnik B, Hackl H, Charoentong P, Tosolini M, Kirilovsky A, et al. ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics. 2009; 25:1091–3.
Article15. Bindea G, Galon J, Mlecnik B. CluePedia Cytoscape plugin: pathway insights using integrated experimental and in silico data. Bioinformatics. 2013; 29:661–3.
Article16. Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, et al. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015; 43(Database issue):D447–52.
Article17. Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003; 13:2498–504.
Article18. Chin CH, Chen SH, Wu HH, Ho CW, Ko MT, Lin CY. CytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst Biol. 2014; 8 Suppl 4:S11.
Article19. Shahian T, Lee GM, Lazic A, Arnold LA, Velusamy P, Roels CM, et al. Inhibition of a viral enzyme by a small-molecule dimer disruptor. Nat Chem Biol. 2009; 5:640–6.
Article20. Tschesche H, Knauper V, Kramer S, Michaelis J, Oberhoff R, Reinke H. Latent collagenase and gelatinase from human neutrophils and their activation. Matrix Suppl. 1992; 1:245–55.21. Williams RN, Parsons SL, Morris TM, Rowlands BJ, Watson SA. Inhibition of matrix metalloproteinase activity and growth of gastric adenocarcinoma cells by an angiotensin converting enzyme inhibitor in in vitro and murine models. Eur J Surg Oncol. 2005; 31:1042–50.
Article22. Chen X, Ji ZL, Chen YZ. TTD: Therapeutic Target Database. Nucleic Acids Res. 2002; 30:412–5.
Article23. Lee CZ, Yao JS, Huang Y, Zhai W, Liu W, Guglielmo BJ, et al. Dose-response effect of tetracyclines on cerebral matrix metalloproteinase-9 after vascular endothelial growth factor hyperstimulation. J Cereb Blood Flow Metab. 2006; 26:1157–64.
Article24. Mendis E, Kim MM, Rajapakse N, Kim SK. Carboxy derivatized glucosamine is a potent inhibitor of matrix metalloproteinase-9 in HT1080 cells. Bioorg Med Chem Lett. 2006; 16:3105–10.
Article25. Rajapakse N, Mendis E, Kim MM, Kim SK. Sulfated glucosamine inhibits MMP-2 and MMP-9 expressions in human fibrosarcoma cells. Bioorg Med Chem. 2007; 15:4891–6.
Article26. Martens E, Leyssen A, Van Aelst I, Fiten P, Piccard H, Hu J, et al. A monoclonal antibody inhibits gelatinase B/MMP-9 by selective binding to part of the catalytic domain and not to the fibronectin or zinc binding domains. Biochim Biophys Acta. 2007; 1770:178–86.
Article27. Ilonen J, Lempainen J, Veijola R. The heterogeneous pathogenesis of type 1 diabetes mellitus. Nat Rev Endocrinol. 2019; 15:635–50.
Article28. Delves PJ, Roitt IM. Roitt’s essential immunology. 12th ed. Chichester: Wiley-Blackwell;2011. p. 546.29. Doumatey AP, Xu H, Huang H, Trivedi NS, Lei L, Elkahloun A, et al. Global gene expression profiling in omental adipose tissue of morbidly obese diabetic African Americans. J Endocrinol Metab. 2015; 5:199–210.
Article30. Shao K, Shen LS, Li HH, Huang S, Zhang Y. Systematic-analysis of mRNA expression profiles in skeletal muscle of patients with type II diabetes: the glucocorticoid was central in pathogenesis. J Cell Physiol. 2018; 233:4068–76.
Article31. Zhong M, Wu Y, Ou W, Huang L, Yang L. Identification of key genes involved in type 2 diabetic islet dysfunction: a bioinformatics study. Biosci Rep. 2019; 39:BSR20182172.
Article32. Van JA, Scholey JW, Konvalinka A. Insights into diabetic kidney disease using urinary proteomics and bioinformatics. J Am Soc Nephrol. 2017; 28:1050–61.
Article33. Gold M, El Khoury J. β-Amyloid, microglia, and the inflammasome in Alzheimer’s disease. Semin Immunopathol. 2015; 37:607–11.
Article34. Morgese MG, Schiavone S, Trabace L. Emerging role of amyloid beta in stress response: implication for depression and diabetes. Eur J Pharmacol. 2017; 817:22–9.
Article35. Carey IM, Critchley JA, DeWilde S, Harris T, Hosking FJ, Cook DG. Risk of infection in type 1 and type 2 diabetes compared with the general population: a matched cohort study. Diabetes Care. 2018; 41:513–21.
Article36. Mendez-Samperio P, de-la-Rosa-Arana JL. Prevention of type 1 diabetes through parasite infection. Immunotherapy. 2015; 7:595–8.
Article37. Kuhtreiber WM, Faustman DL. BCG therapy for type 1 diabetes: restoration of balanced immunity and metabolism. Trends Endocrinol Metab. 2019; 30:80–92.
Article38. Stienstra R, van Diepen JA, Tack CJ, Zaki MH, van de Veerdonk FL, Perera D, et al. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci U S A. 2011; 108:15324–9.
Article39. He Y, Hara H, Nunez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci. 2016; 41:1012–21.
Article40. Wan Z, Fan Y, Liu X, Xue J, Han Z, Zhu C, et al. NLRP3 inflammasome promotes diabetes-induced endothelial inflammation and atherosclerosis. Diabetes Metab Syndr Obes. 2019; 12:1931–42.41. Wu KK. Deep fibromatosis of the foot with metatarsal involvement. J Foot Surg. 1989; 28:586–90.42. Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat Immunol. 2010; 11:897–904.
Article43. Rehli M, Krause SW, Andreesen R. Molecular characterization of the gene for human cartilage gp-39 (CHI3L1), a member of the chitinase protein family and marker for late stages of macrophage differentiation. Genomics. 1997; 43:221–5.
Article44. Batinic K, Hobaus C, Grujicic M, Steffan A, Jelic F, Lorant D, et al. YKL-40 is elevated in patients with peripheral arterial disease and diabetes or pre-diabetes. Atherosclerosis. 2012; 222:557–63.
Article45. Gorgens SW, Eckardt K, Elsen M, Tennagels N, Eckel J. Chitinase-3-like protein 1 protects skeletal muscle from TNFα-induced inflammation and insulin resistance. Biochem J. 2014; 459:479–88.
Article46. Moser B, Clark-Lewis I, Zwahlen R, Baggiolini M. Neutrophilactivating properties of the melanoma growth-stimulatory activity. J Exp Med. 1990; 171:1797–802.
Article47. Schumacher C, Clark-Lewis I, Baggiolini M, Moser B. Highand low-affinity binding of GRO alpha and neutrophil-activating peptide 2 to interleukin 8 receptors on human neutrophils. Proc Natl Acad Sci U S A. 1992; 89:10542–6.48. Silva RL, Lopes AH, Guimaraes RM, Cunha TM. CXCL1/ CXCR2 signaling in pathological pain: role in peripheral and central sensitization. Neurobiol Dis. 2017; 105:109–16.49. Opdenakker G, Van den Steen PE, Dubois B, Nelissen I, Van Coillie E, Masure S, et al. Gelatinase B functions as regulator and effector in leukocyte biology. J Leukoc Biol. 2001; 69:851–9.50. Graham KL, Krishnamurthy B, Fynch S, Mollah ZU, Slattery R, Santamaria P, et al. Autoreactive cytotoxic T lymphocytes acquire higher expression of cytotoxic effector markers in the islets of NOD mice after priming in pancreatic lymph nodes. Am J Pathol. 2011; 178:2716–25.
Article51. Kobayashi M, Kaneko-Koike C, Abiru N, Uchida T, Akazawa S, Nakamura K, et al. Genetic deletion of granzyme B does not confer resistance to the development of spontaneous diabetes in non-obese diabetic mice. Clin Exp Immunol. 2013; 173:411–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparative co-expression analysis of RNA-Seq transcriptome revealing key genes, miRNA and transcription factor in distinct metabolic pathways in diabetic nerve, eye, and kidney disease
- Imbalance of Innate and Adaptive Immunity in Esophageal Achalasia
- Identification of multiple hub genes in acute kidney injury after kidney transplantation by bioinformatics analysis
- A network-biology approach for identification of key genes and pathways involved in malignant peritoneal mesothelioma
- Identification of Hub Genes in the Pathogenesis of Ischemic Stroke Based on Bioinformatics Analysis