J Korean Med Sci.
2022 May;37(21):e168. 10.3346/jkms.2022.37.e168.
Positivity of Rapid Antigen Testing for SARS-CoV-2 With Serial Followed-up Nasopharyngeal Swabs in Hospitalized Patients due to COVID-19
- Affiliations
-
- 1Department of Laboratory Medicine, Chung-Ang University College of Medicine, Seoul, Korea
- 2Department of Pulmonary Medicine, Hyundae General Hospital, Chung-Ang University, Namyangju, Korea
- 3Department of Orthopaedic Surgery, Chung-Ang University Hospital, Seoul, Korea
- 4Department of Orthopaedic Surgery, Hyundae General Hospital, Chung-Ang University, Namyangju, Korea
- 5Department of Internal Medicine, Hyundae General Hospital, Chung-Ang University, Namyangju, Korea
- 6Department of Pediatrics, Chung-Ang University Hospital, Seoul, Korea
- KMID: 2530113
- DOI: http://doi.org/10.3346/jkms.2022.37.e168
Abstract
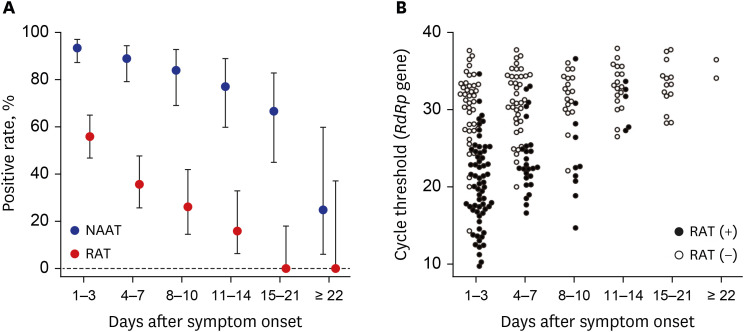
- Despite the accuracy of nucleic acid amplification tests (NAATs), rapid antigen tests (RATs) for severe acute respiratory syndrome coronavirus-2 are widely used as point-of-care tests. A total of 282 pairs of reverse transcription-polymerase chain reaction and Standard Q COVID-19 Ag tests were serially conducted for 68 patients every 3–4 days until their discharge. Through a field evaluation of RATs using direct nasopharyngeal swabs, the sensitivities were 84.6% and 87.3% for E and RNA-dependent RNA polymerase (RdRp) genes, respectively, for specimens with cycle thresholds (Cts) < 25. The Ct values of E and RdRp genes for 95% detection rates by RATs were 16.9 and 18.1, respectively. The sensitivity of RAT was 48.4% after the onset of symptoms, which was not sufficient. RAT positivity gradually decreased with increased time after symptom onset and had continuously lower sensitivity than NAATs.
Figure
Reference
-
1. Hong KH, Lee SW, Kim TS, Huh HJ, Lee J, Kim SY, et al. Guidelines for laboratory diagnosis of coronavirus disease 2019 (COVID-19) in Korea. Ann Lab Med. 2020; 40(5):351–360. PMID: 32237288.2. Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020; 25(3):2000045.3. Kim MC, Cui C, Shin KR, Bae JY, Kweon OJ, Lee MK, et al. Duration of culturable SARS-CoV-2 in hospitalized patients with COVID-19. N Engl J Med. 2021; 384(7):671–673. PMID: 33503337.4. Somborac Bačura A, Dorotić M, Grošić L, Džimbeg M, Dodig S. Current status of the lateral flow immunoassay for the detection of SARS-CoV-2 in nasopharyngeal swabs. Biochem Med (Zagreb). 2021; 31(2):020601. PMID: 34140830.5. Surasi K, Cummings KJ, Hanson C, Morris MK, Salas M, Seftel D, et al. Effectiveness of Abbott BinaxNOW Rapid Antigen Test for detection of SARS-CoV-2 infections in outbreak among horse racetrack workers, California, USA. Emerg Infect Dis. 2021; 27(11):2761–2767. PMID: 34469287.6. Oh SM, Jeong H, Chang E, Choe PG, Kang CK, Park WB, et al. Clinical application of the Standard Q COVID-19 Ag test for the detection of SARS-CoV-2 infection. J Korean Med Sci. 2021; 36(14):e101. PMID: 33847084.7. Chaimayo C, Kaewnaphan B, Tanlieng N, Athipanyasilp N, Sirijatuphat R, Chayakulkeeree M, et al. Rapid SARS-CoV-2 antigen detection assay in comparison with real-time RT-PCR assay for laboratory diagnosis of COVID-19 in Thailand. Virol J. 2020; 17(1):177. PMID: 33187528.8. Kim HW, Park M, Lee JH. Clinical evaluation of the rapid STANDARD Q COVID-19 Ag test for the screening of severe acute respiratory syndrome coronavirus 2. Ann Lab Med. 2022; 42(1):100–104. PMID: 34374355.9. Amer RM, Samir M, Gaber OA, El-Deeb NA, Abdelmoaty AA, Ahmed AA, et al. Diagnostic performance of rapid antigen test for COVID-19 and the effect of viral load, sampling time, subject’s clinical and laboratory parameters on test accuracy. J Infect Public Health. 2021; 14(10):1446–1453. PMID: 34175237.10. Korenkov M, Poopalasingam N, Madler M, Vanshylla K, Eggeling R, Wirtz M, et al. Evaluation of a rapid antigen test to detect SARS-CoV-2 infection and identify potentially infectious individuals. J Clin Microbiol. 2021; 59(9):e0089621. PMID: 34213977.11. Nalumansi A, Lutalo T, Kayiwa J, Watera C, Balinandi S, Kiconco J, et al. Field evaluation of the performance of a SARS-CoV-2 antigen rapid diagnostic test in Uganda using nasopharyngeal samples. Int J Infect Dis. 2021; 104:282–286. PMID: 33130198.12. Ristić M, Nikolić N, Čabarkapa V, Turkulov V, Petrović V. Validation of the STANDARD Q COVID-19 antigen test in Vojvodina, Serbia. PLoS One. 2021; 16(2):e0247606. PMID: 33617597.13. Peña-Rodríguez M, Viera-Segura O, García-Chagollán M, Zepeda-Nuño JS, Muñoz-Valle JF, Mora-Mora J, et al. Performance evaluation of a lateral flow assay for nasopharyngeal antigen detection for SARS-CoV-2 diagnosis. J Clin Lab Anal. 2021; 35(5):e23745. PMID: 33675086.14. Berger A, Nsoga MT, Perez-Rodriguez FJ, Aad YA, Sattonnet-Roche P, Gayet-Ageron A, et al. Diagnostic accuracy of two commercial SARS-CoV-2 antigen-detecting rapid tests at the point of care in community-based testing centers. PLoS One. 2021; 16(3):e0248921. PMID: 33788882.15. Routsias JG, Mavrouli M, Tsoplou P, Dioikitopoulou K, Tsakris A. Diagnostic performance of rapid antigen tests (RATs) for SARS-CoV-2 and their efficacy in monitoring the infectiousness of COVID-19 patients. Sci Rep. 2021; 11(1):22863. PMID: 34819567.16. Zhou J, Otter JA, Price JR, Cimpeanu C, Meno Garcia D, Kinross J, et al. Investigating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) surface and air contamination in an acute healthcare setting during the peak of the coronavirus disease 2019 (COVID-19) pandemic in London. Clin Infect Dis. 2021; 73(7):e1870–e1877. PMID: 32634826.17. Kohmer N, Toptan T, Pallas C, Karaca O, Pfeiffer A, Westhaus S, et al. The comparative clinical performance of four SARS-CoV-2 rapid antigen tests and their correlation to infectivity in vitro. J Clin Med. 2021; 10(2):328. PMID: 33477365.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Serial Screening for SARS-CoV-2 in Rectal Swabs of Symptomatic COVID-19 Patients
- Challenges of Scaling Up SARS-CoV-2 Rapid Antigen Tests
- Performance of STANDARD™ M10 SARS-CoV-2 Assay for the Diagnosis of COVID-19 from a Nasopharyngeal Swab
- Response to positive patients with COVID-19 self-test visiting the emergency department
- Laboratory Diagnosis of COVID-19 in Korea