Korean J Physiol Pharmacol.
2022 May;26(3):175-182. 10.4196/kjpp.2022.26.3.175.
Lysophosphatidylcholine induces azurophil granule translocation via Rho/Rho kinase/F-actin polymerization in human neutrophils
- Affiliations
-
- 1Department of Pharmacology, Hallym University College of Medicine, Chuncheon 24252, Korea
- KMID: 2529400
- DOI: http://doi.org/10.4196/kjpp.2022.26.3.175
Abstract
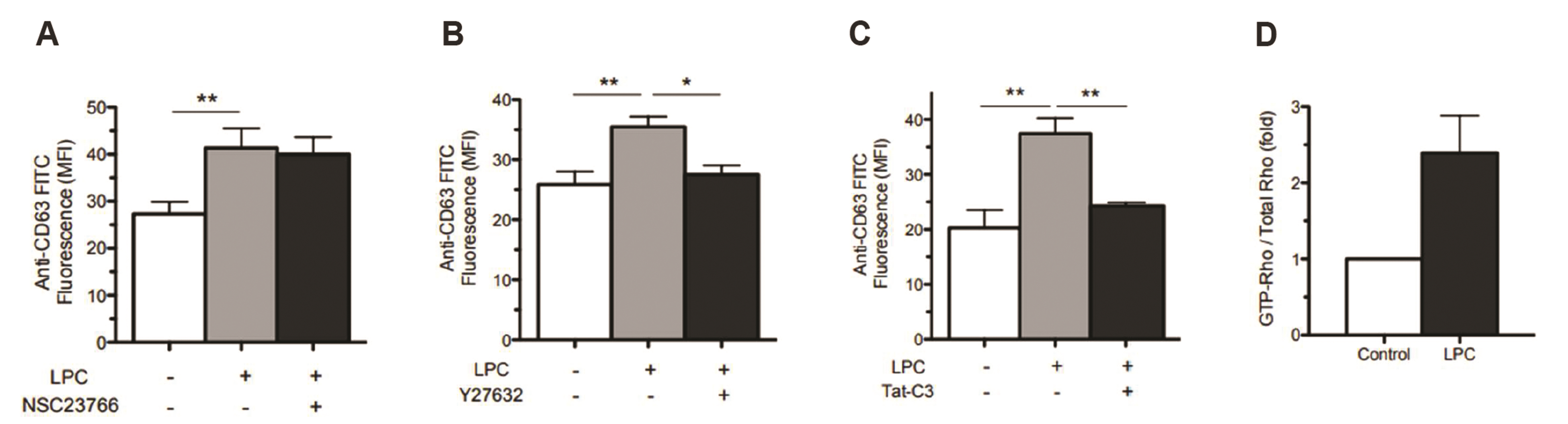
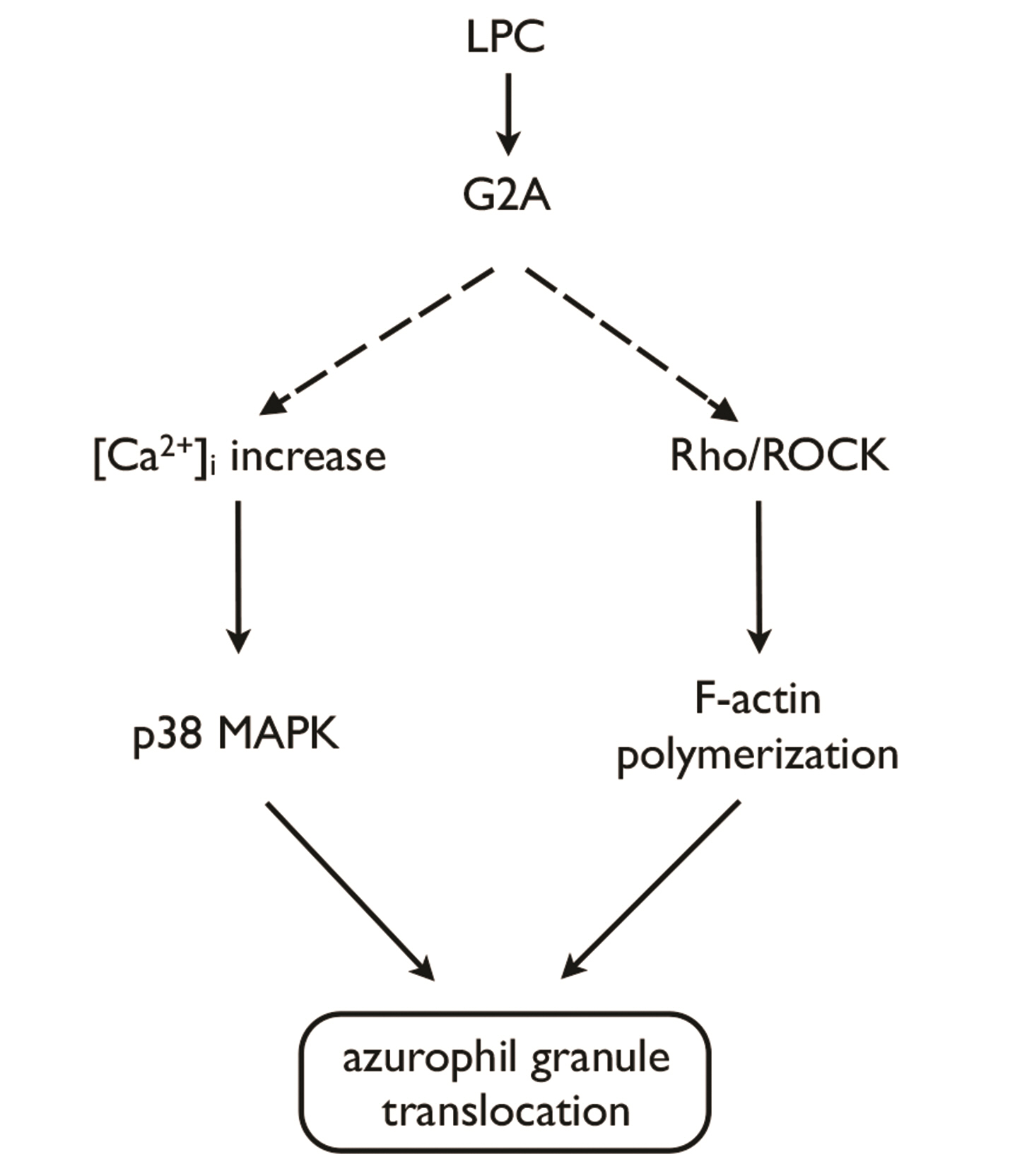
- Translocation of azurophil granules is pivotal for bactericidal activity of neutrophils, the first-line defense cells against pathogens. Previously, we reported that lysophosphatidylcholine (LPC), an endogenous lipid, enhances bactericidal activity of human neutrophils via increasing translocation of azurophil granules. However, the precise mechanism of LPC-induced azurophil granule translocation was not fully understood. Treatment of neutrophil with LPC significantly increased CD63 (an azurophil granule marker) surface expression. Interestingly, cytochalasin B, an inhibitor of action polymerization, blocked LPC-induced CD63 surface expression. LPC increased F-actin polymerization. LPC-induced CD63 surface expression was inhibited by both a Rho specific inhibitor, Tat-C3 exoenzyme, and a Rho kinase (ROCK) inhibitor, Y27632 which also inhibited LPC-induced F-actin polymerization. LPC induced Rho-GTP activation. NSC23766, a Rac inhibitor, however, did not affect LPC-induced CD63 surface expression. Theses results suggest a novel regulatory mechanism for azurophil granule translocation where LPC induces translocation of azurophil granules via Rho/ROCK/F-actin polymerization pathway.
Figure
Reference
-
1. Segal AW. 2005; How neutrophils kill microbes. Annu Rev Immunol. 23:197–223. DOI: 10.1146/annurev.immunol.23.021704.115653. PMID: 15771570. PMCID: PMC2092448.
Article2. Nauseef WM. 2007; How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev. 219:88–102. DOI: 10.1111/j.1600-065X.2007.00550.x. PMID: 17850484.
Article3. Borregaard N, Cowland JB. 1997; Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 89:3503–3521. DOI: 10.1182/blood.V89.10.3503.3503_3503_3521. PMID: 9160655.
Article4. Häger M, Cowland JB, Borregaard N. 2010; Neutrophil granules in health and disease. J Intern Med. 268:25–34. DOI: 10.1111/j.1365-2796.2010.02237.x. PMID: 20497300.
Article5. Lew PD, Monod A, Waldvogel FA, Dewald B, Baggiolini M, Pozzan T. 1986; Quantitative analysis of the cytosolic free calcium dependency of exocytosis from three subcellular compartments in intact human neutrophils. J Cell Biol. 102:2197–2204. DOI: 10.1083/jcb.102.6.2197. PMID: 3011810. PMCID: PMC2114244.
Article6. Bentwood BJ, Henson PM. 1980; The sequential release of granule constitutents from human neutrophils. J Immunol. 124:855–862. PMID: 6153206.7. Jog NR, Rane MJ, Lominadze G, Luerman GC, Ward RA, McLeish KR. 2007; The actin cytoskeleton regulates exocytosis of all neutrophil granule subsets. Am J Physiol Cell Physiol. 292:C1690–C1700. DOI: 10.1152/ajpcell.00384.2006. PMID: 17202227.
Article8. Takai Y, Sasaki T, Matozaki T. 2001; Small GTP-binding proteins. Physiol Rev. 81:153–208. DOI: 10.1152/physrev.2001.81.1.153. PMID: 11152757.
Article9. Ridley AJ. 2001; Rho proteins: linking signaling with membrane trafficking. Traffic. 2:303–310. DOI: 10.1034/j.1600-0854.2001.002005303.x. PMID: 11350626.
Article10. Wheeler AP, Ridley AJ. 2004; Why three Rho proteins? RhoA, RhoB, RhoC, and cell motility. Exp Cell Res. 301:43–49. DOI: 10.1016/j.yexcr.2004.08.012. PMID: 15501444.
Article11. Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. 2003; Cell migration: integrating signals from front to back. Science. 302:1704–1709. DOI: 10.1126/science.1092053. PMID: 14657486.
Article12. Spiering D, Hodgson L. 2011; Dynamics of the Rho-family small GTPases in actin regulation and motility. Cell Adh Migr. 5:170–180. DOI: 10.4161/cam.5.2.14403. PMID: 21178402. PMCID: PMC3084983.
Article13. Guan X, Guan X, Dong C, Jiao Z. 2020; Rho GTPases and related signaling complexes in cell migration and invasion. Exp Cell Res. 388:111824. DOI: 10.1016/j.yexcr.2020.111824. PMID: 31926148.
Article14. Fessler MB, Arndt PG, Just I, Nick JA, Malcolm KC, Worthen GS. 2007; Dual role for RhoA in suppression and induction of cytokines in the human neutrophil. Blood. 109:1248–1256. DOI: 10.1182/blood-2006-03-012898. PMID: 17018860. PMCID: PMC1785129.
Article15. Abdel-Latif D, Steward M, Macdonald DL, Francis GA, Dinauer MC, Lacy P. 2004; Rac2 is critical for neutrophil primary granule exocytosis. Blood. 104:832–839. DOI: 10.1182/blood-2003-07-2624. PMID: 15073033.
Article16. Lacy P, Eitzen G. 2008; Control of granule exocytosis in neutrophils. Front Biosci. 13:5559–5570. DOI: 10.2741/3099. PMID: 18508605.
Article17. Eitzen G, Lo AN, Mitchell T, Kim JD, Chao DV, Lacy P. 2011; Proteomic analysis of secretagogue-stimulated neutrophils implicates a role for actin and actin-interacting proteins in Rac2-mediated granule exocytosis. Proteome Sci. 9:70. DOI: 10.1186/1477-5956-9-70. PMID: 22081935. PMCID: PMC3379032.
Article18. Johnson JL, Brzezinska AA, Tolmachova T, Munafo DB, Ellis BA, Seabra MC, Hong H, Catz SD. 2010; Rab27a and Rab27b regulate neutrophil azurophilic granule exocytosis and NADPH oxidase activity by independent mechanisms. Traffic. 11:533–547. DOI: 10.1111/j.1600-0854.2009.01029.x. PMID: 20028487. PMCID: PMC2937183.
Article19. Englberger W, Bitter-Suermann D, Hadding U. 1987; Influence of lysophospholipids and PAF on the oxidative burst of PMNL. Int J Immunopharmacol. 9:275–282. DOI: 10.1016/0192-0561(87)90051-8. PMID: 3038761.
Article20. Frasch SC, Zemski-Berry K, Murphy RC, Borregaard N, Henson PM, Bratton DL. 2007; Lysophospholipids of different classes mobilize neutrophil secretory vesicles and induce redundant signaling through G2A. J Immunol. 178:6540–6548. DOI: 10.4049/jimmunol.178.10.6540. PMID: 17475884.
Article21. Khan SY, McLaughlin NJ, Kelher MR, Eckels P, Gamboni-Robertson F, Banerjee A, Silliman CC. 2010; Lysophosphatidylcholines activate G2A inducing Gαi-1-/Gαq/₁₁- Ca2+ flux, Gβγ-Hck activation and clathrin/β-arrestin-1/GRK6 recruitment in PMNs. Biochem J. 432:35–45. DOI: 10.1042/BJ20091087. PMID: 20799926. PMCID: PMC3131183.
Article22. Lin P, Welch EJ, Gao XP, Malik AB, Ye RD. 2005; Lysophosphatidylcholine modulates neutrophil oxidant production through elevation of cyclic AMP. J Immunol. 174:2981–2989. DOI: 10.4049/jimmunol.174.5.2981. PMID: 15728511.
Article23. Silliman CC, Elzi DJ, Ambruso DR, Musters RJ, Hamiel C, Harbeck RJ, Paterson AJ, Bjornsen AJ, Wyman TH, Kelher M, England KM, McLaughlin-Malaxecheberria N, Barnett CC, Aiboshi J, Bannerjee A. 2003; Lysophosphatidylcholines prime the NADPH oxidase and stimulate multiple neutrophil functions through changes in cytosolic calcium. J Leukoc Biol. 73:511–524. DOI: 10.1189/jlb.0402179. PMID: 12660226.
Article24. Yan JJ, Jung JS, Lee JE, Lee J, Huh SO, Kim HS, Jung KC, Cho JY, Nam JS, Suh HW, Kim YH, Song DK. 2004; Therapeutic effects of lysophosphatidylcholine in experimental sepsis. Nat Med. 10:161–167. DOI: 10.1038/nm989. PMID: 14716308.
Article25. Kelher MR, McLaughlin NJ, Banerjee A, Elzi DJ, Gamboni F, Khan SY, Meng X, Mitra S, Silliman CC. 2017; LysoPCs induce Hck- and PKCδ-mediated activation of PKCγ causing p47phox phosphorylation and membrane translocation in neutrophils. J Leukoc Biol. 101:261–273. DOI: 10.1189/jlb.3A0813-420RRR. PMID: 27531930. PMCID: PMC5166440.
Article26. Song MH, Gupta A, Kim HO, Oh K. 2021; Lysophosphatidylcholine aggravates contact hypersensitivity by promoting neutrophil infiltration and IL17 expression. BMB Rep. 54:203–208. DOI: 10.5483/BMBRep.2021.54.4.193. PMID: 33172544. PMCID: PMC8093940.
Article27. Hong CW, Kim TK, Ham HY, Nam JS, Kim YH, Zheng H, Pang B, Min TK, Jung JS, Lee SN, Cho HJ, Kim EJ, Hong IH, Kang TC, Lee J, Oh SB, Jung SJ, Kim SJ, Song DK. 2010; Lysophosphatidylcholine increases neutrophil bactericidal activity by enhancement of azurophil granule-phagosome fusion via glycine.GlyR alpha 2/TRPM2/p38 MAPK signaling. J Immunol. 184:4401–4413. DOI: 10.4049/jimmunol.0902814. PMID: 20237295.
Article28. Kim JS, Diebold BA, Kim JI, Kim J, Lee JY, Park JB. 2004; Rho is involved in superoxide formation during phagocytosis of opsonized zymosans. J Biol Chem. 279:21589–21597. DOI: 10.1074/jbc.M308386200. PMID: 14970220.
Article29. Kuijpers TW, Tool AT, van der Schoot CE, Ginsel LA, Onderwater JJ, Roos D, Verhoeven AJ. 1991; Membrane surface antigen expression on neutrophils: a reappraisal of the use of surface markers for neutrophil activation. Blood. 78:1105–1111. DOI: 10.1182/blood.V78.4.1105.bloodjournal7841105. PMID: 1907873.
Article30. Mitchell T, Lo A, Logan MR, Lacy P, Eitzen G. 2008; Primary granule exocytosis in human neutrophils is regulated by Rac-dependent actin remodeling. Am J Physiol Cell Physiol. 295:C1354–C1365. DOI: 10.1152/ajpcell.00239.2008. PMID: 18799653. PMCID: PMC2878813.
Article31. Lin P, Ye RD. 2003; The lysophospholipid receptor G2A activates a specific combination of G proteins and promotes apoptosis. J Biol Chem. 278:14379–14386. DOI: 10.1074/jbc.M209101200. PMID: 12586833.
Article32. Yang LV, Radu CG, Wang L, Riedinger M, Witte ON. 2005; Gi-independent macrophage chemotaxis to lysophosphatidylcholine via the immunoregulatory GPCR G2A. Blood. 105:1127–1134. DOI: 10.1182/blood-2004-05-1916. PMID: 15383458.
Article33. Kabarowski JH, Feramisco JD, Le LQ, Gu JL, Luoh SW, Simon MI, Witte ON. 2000; Direct genetic demonstration of G alpha 13 coupling to the orphan G protein-coupled receptor G2A leading to RhoA-dependent actin rearrangement. Proc Natl Acad Sci U S A. 97:12109–12114. DOI: 10.1073/pnas.97.22.12109. PMID: 11050239. PMCID: PMC17302.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Glycine induces enhancement of bactericidal activity of neutrophils
- The role of Rho GTPases in the regulation of the rearrangement of actin cytoskeleton and cell movement
- Rho-kinase and Insulin Signaling
- Rho-Associated Kinase 2 Polymorphism of Vasospastic Angina in Korean Population
- Clathrin and Lipid Raft-dependent Internalization of Porphyromonas gingivalis in Endothelial Cells