Korean J Physiol Pharmacol.
2022 May;26(3):145-155. 10.4196/kjpp.2022.26.3.145.
Chemosensitizing effect and mechanism of imperatorin on the anti-tumor activity of doxorubicin in tumor cells and transplantation tumor model
- Affiliations
-
- 1Key Laboratory of Modern Preparation of Chinese Medicine, Ministry of Education, Jiangxi University of Chinese Medicine, Nanchang 330004, China
- 2Jiangxi Provincial Key Laboratory of Drug Design and Evaluation, School of Pharmacy, Nanchang 330013, China
- KMID: 2529397
- DOI: http://doi.org/10.4196/kjpp.2022.26.3.145
Abstract
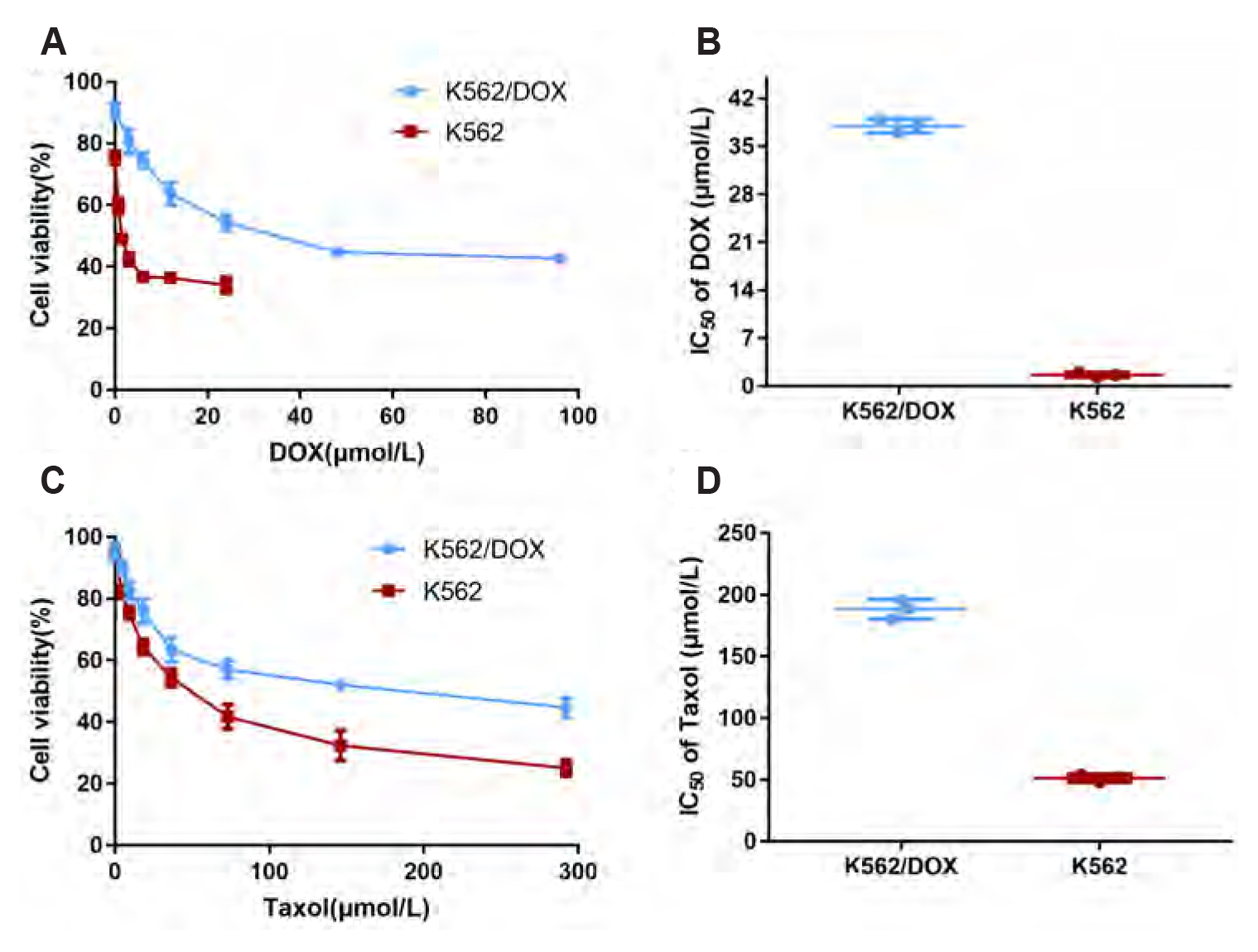
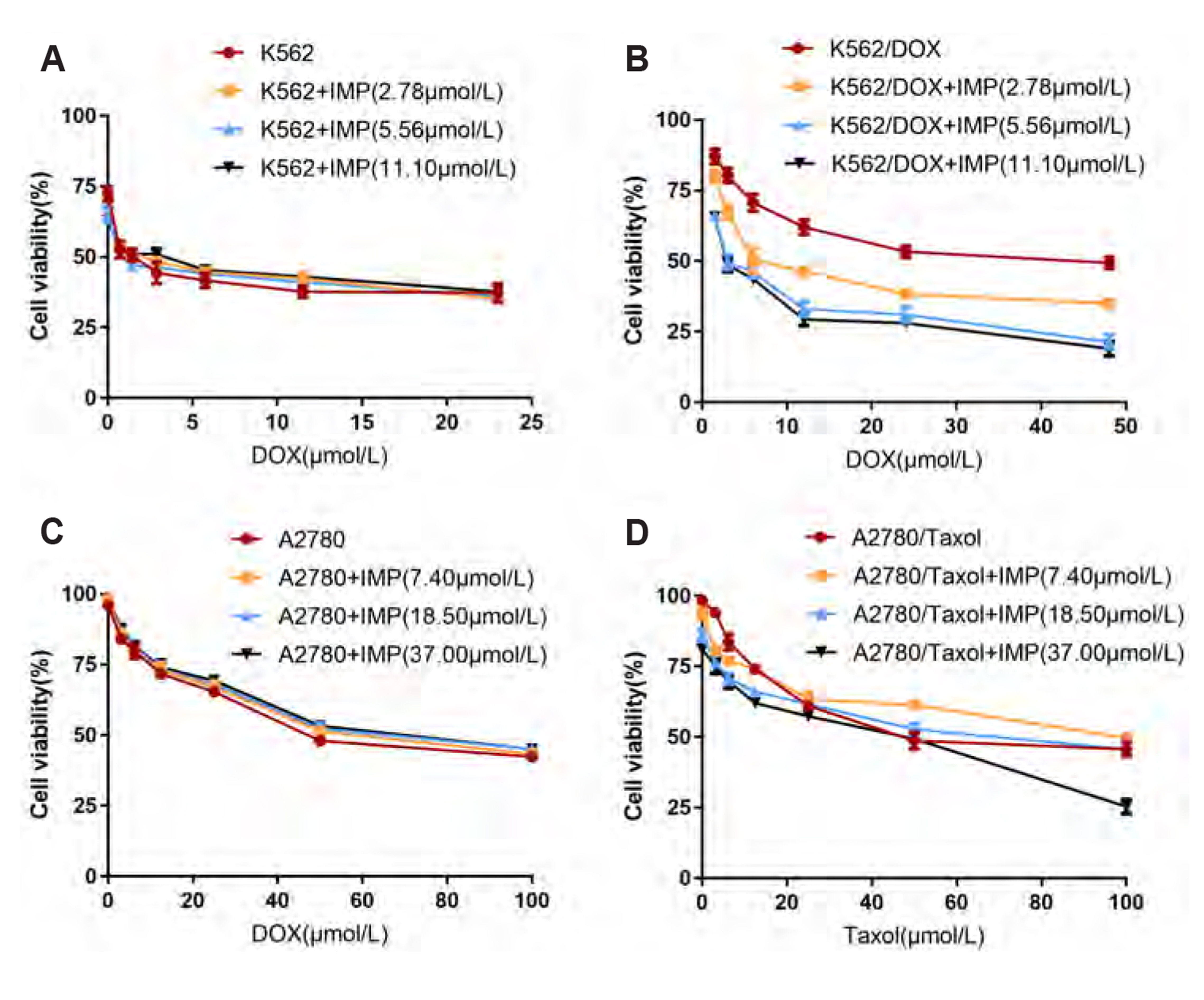
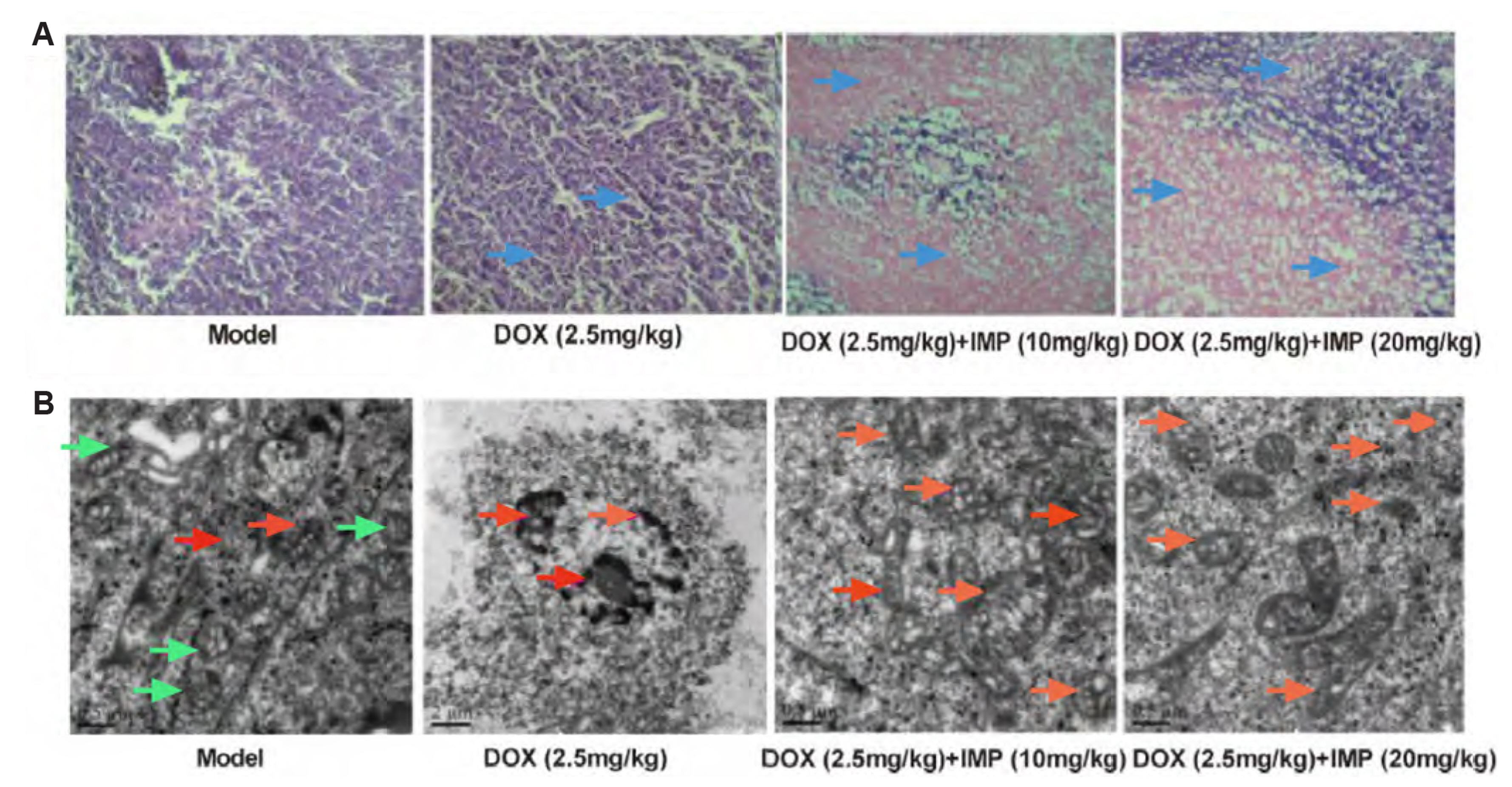
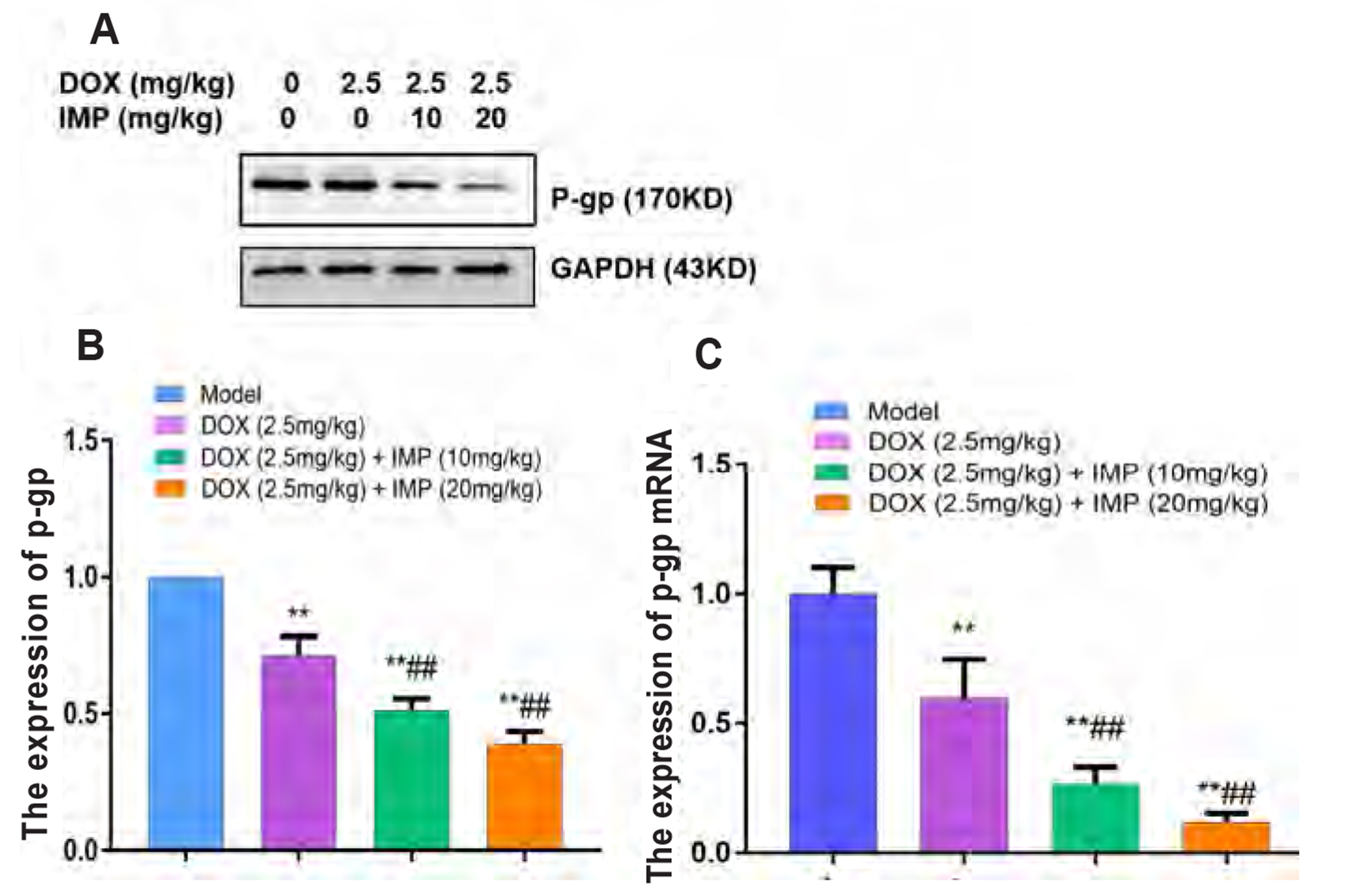
- Multidrug resistance of tumors has been a severe obstacle to the success of cancer chemotherapy. The study wants to investigate the reversal effects of imperatorin (IMP) on doxorubicin (DOX) resistance in K562/DOX leukemia cells, A2780/Taxol cells and in NOD/SCID mice, to explore the possible molecular mechanisms. K562/ DOX and A2780/Taxol cells were treated with various concentrations of DOX and Taol with or without different concentrations of IMP, respectively. K562/DOX xenograft model was used to assess anti-tumor effect of IMP combined with DOX. MTT assay, Rhodamine 123 efflux assay, RT-PCR, and Western blot analysis were determined in vivo and in vitro. Results showed that IMP significantly enhanced the cytotoxicity of DOX and Taxol toward corresponding resistance cells. In vivo results illustrated both the tumor volume and tumor weight were significantly decreased after 2-week treatment with IMP combined with DOX compared to the DOX alone group. Western blotting and RT-PCR analyses indicated that IMP downregulated the expression of P-gp in K562/DOX xenograft tumors in NOD/SCID mice. We also evaluated glycolysis and glutamine metabolism in K562/DOX cells by measuring glucose consumption and lactate production. The results revealed that IMP could significantly reduce the glucose consumption and lactate production of K562/DOX cells. Furthermore, IMP could also remarkably repress the glutamine consumption, α-KG and ATP production of K562/DOX cells. Thus, IMP may sensitize K562/DOX cells to DOX and enhance the antitumor effect of DOX in K562/DOX xenograft tumors in NOD/SCID mice. IMP may be an adjuvant therapy to mitigate the multidrug resistance in leukemia chemotherapy.
Keyword
Figure
Reference
-
1. Abad MJ, de las Heras B, Silván AM, Pascual R, Bermejo P, Rodriquez B, Villar AM. 2001; Effects of furocoumarins from Cachrys trifida on some macrophage functions. J Pharm Pharmacol. 53:1163–1168. DOI: 10.1211/0022357011776432. PMID: 11518028.
Article2. Al-Ali AAA, Nielsen RB, Steffansen B, Holm R, Nielsen CU. 2019; Nonionic surfactants modulate the transport activity of ATP-binding cassette (ABC) transporters and solute carriers (SLC): relevance to oral drug absorption. Int J Pharm. 566:410–433. DOI: 10.1016/j.ijpharm.2019.05.033. PMID: 31125713.
Article3. Al-Mohizea AM, Al-Jenoobi FI, Alam MA. 2015; Rhodamine-123: a p-glycoprotein marker complex with sodium lauryl sulfate. Pak J Pharm Sci. 28:617–622. PMID: 25730814.4. Baek NI, Ahn EM, Kim HY, Park YD. 2000; Furanocoumarins from the root of Angelica dahurica. Arch Pharm Res. 23:467–470. DOI: 10.1007/BF02976574. PMID: 11059825.5. Breier A, Barancík M, Sulová Z, Uhrík B. 2005; P-glycoprotein--implications of metabolism of neoplastic cells and cancer therapy. Curr Cancer Drug Targets. 5:457–468. DOI: 10.2174/1568009054863636. PMID: 16178819.
Article6. Dong W, Guan X, Liao Z, Lu X, Liang X, Zhu W. 2016; In vitro study on affinity of coumarins in Angelicae Dahuricae Radix and P-gp. Chin Tradit Herb Drugs. 47:2893–2896. https://www.tiprpress.com/zcy/article/abstract/20161620. Chinese.7. Duran GE, Wang YC, Moisan F, Francisco EB, Sikic BI. 2017; Decreased levels of baseline and drug-induced tubulin polymerisation are hallmarks of resistance to taxanes in ovarian cancer cells and are associated with epithelial-to-mesenchymal transition. Br J Cancer. 116:1318–1328. DOI: 10.1038/bjc.2017.102. PMID: 28399108. PMCID: PMC5482726.
Article8. Fan L, Jin B, Zhang S, Song C, Li Q. 2016; Stimuli-free programmable drug release for combination chemo-therapy. Nanoscale. 8:12553–12559. DOI: 10.1039/C5NR06305A. PMID: 26554664.
Article9. Fang S, Zhu W, Zhang Y, Shu Y, Liu P. 2012; Paeoniflorin modulates multidrug resistance of a human gastric cancer cell line via the inhibition of NF-κB activation. Mol Med Rep. 5:351–356. DOI: 10.3892/mmr.2011.652. PMID: 22051979.10. Ganta S, Amiji M. 2009; Coadministration of paclitaxel and curcumin in nanoemulsion formulations to overcome multidrug resistance in tumor cells. Mol Pharm. 6:928–939. DOI: 10.1021/mp800240j. PMID: 19278222.
Article11. Ge C, Cao B, Feng D, Zhou F, Zhang J, Yang N, Feng S, Wang G, Aa J. 2017; The down-regulation of SLC7A11 enhances ROS induced P-gp over-expression and drug resistance in MCF-7 breast cancer cells. Sci Rep. 7:3791. DOI: 10.1038/s41598-017-03881-9. PMID: 28630426. PMCID: PMC5476638. PMID: 13e5613b1d2141a3bc3ada2ae6cb0dbe.
Article12. Gou Q, Liu L, Wang C, Wu Q, Sun L, Yang X, Xie Y, Li P, Gong C. 2015; Polymeric nanoassemblies entrapping curcumin overcome multidrug resistance in ovarian cancer. Colloids Surf B Biointerfaces. 126:26–34. DOI: 10.1016/j.colsurfb.2014.12.012. PMID: 25543980.
Article13. Gottesman MM, Ling V. 2006; The molecular basis of multidrug resistance in cancer: the early years of P-glycoprotein research. FEBS Lett. 580:998–1009. DOI: 10.1016/j.febslet.2005.12.060. PMID: 16405967.
Article14. Guan X, Yu S, Liao Z, Zhu W, Zhao G, Luo Y, Liang X. 2015; Effects of 10 furanocoumarins in Angelica dahurica on intestinal transport of vincristine. Chin Tradit Herb Drugs. 46:2117–2121. https://www.tiprpress.com/zcy/article/abstract/20151417. Chinese. DOI: 10.1016/j.febslet.2005.12.060.15. Hait WN, Yang JM. 2005; Clinical management of recurrent breast cancer: development of multidrug resistance (MDR) and strategies to circumvent it. Semin Oncol. 32(6 Suppl 7):S16–S21. DOI: 10.1053/j.seminoncol.2005.09.011. PMID: 16360718.
Article16. He L, Liu GQ. 2002; Interaction of multidrug resistance reversal agents with P-glycoprotein ATPase activity on blood-brain barrier. Acta Pharmacol Sin. 23:423–429. PMID: 11978192.17. Jia Y, Sun S, Gao X, Cui X. 2018; Expression levels of TUBB3, ERCC1 and P-gp in ovarian cancer tissues and adjacent normal tissues and their clinical significance. J BUON. 23:1390–1395. PMID: 30570863.18. Kocibalova Z, Guzyova M, Imrichova D, Sulova Z, Breier A. 2018; Overexpression of the ABCB1 drug transporter in acute myeloid leukemia cells is associated with downregulation of latrophilin-1. Gen Physiol Biophys. 37:353–357. DOI: 10.4149/gpb_2018008. PMID: 29938681.
Article19. Kozioł E, Skalicka-Woźniak K. 2016; Imperatorin-pharmacological meaning and analytical clues: profound investigation. Phytochem Rev. 15:627–649. DOI: 10.1007/s11101-016-9456-2. PMID: 27453708. PMCID: PMC4939159.
Article20. Li J, Qin F, Yang P. 2002; Reversal of multidrug resistance in human K562/ADM cell line by dauricine. J Dalian Med Univ. 24:94–96. http://europepmc.org/article/CBA/370966. Chinese.
Article21. Liang X, Dong W, Zhang J, Zhao G, Luo Y, Liao Z. 2016. Quantitative method study on transport mechanism of puerarin, berberine hydrochloride and paeoniflorin. Paper presented at: International Conference on Biotechnology and Medical Science. 2016 Apr 16-17; Nanjing, China. https://www.worldscientific.com/doi/abs/10.1142/9789813145870_0039. DOI: 10.1142/9789813145870_0039. PMCID: PMC5093998.
Article22. Li Z, Tan S, Li S, Shen Q, Wang K. 2017; Cancer drug delivery in the nano era: an overview and perspectives (review). Oncol Rep. 38:611–624. DOI: 10.3892/or.2017.5718. PMID: 28627697. PMCID: PMC5562049.23. Li Z, Tang T, Liang X, Zhu J. 2019; Imperatorin reverses multidrug resistance of Huma Oophoroma Cancer Cell A2780/Taxol and its mechanism. Pharmacol Clin Chin Mater Med. 35:31–33. http://www.cglhub.com/auto/db/detail.aspx?db=950015&rid=55375120&agfi=0&cls=0&uni=True&cid=0&showgp=True&prec=False&md=93&pd=6&msd=93&psd=6&mdd=93&pdd=6&count=10&reds=%E9%99%88%E5%86%AC%E5%BB%BA. Chinese.24. Li Z, Wang C, Tang T, Liang X, Zhu J. 2019; Effect of imperatorin on the content of Rho 123 in the drug-resistant cell suspension of tumor by HPLC-FLD. Chin J Pharm Anal. 9:1567–1573. http://html.rhhz.net/ywfxzz/html/20190904.htm. Chinese.25. Ma X, Hu M, Wang H, Li J. 2018; Discovery of traditional Chinese medicine monomers and their synthetic intermediates, analogs or derivatives for battling P-gp-mediated multi-drug resistance. Eur J Med Chem. 159:381–392. DOI: 10.1016/j.ejmech.2018.09.061. PMID: 30308411.
Article26. Mao X, Si J, Huang Q, Sun X, Zhang Q, Shen Y, Tang J, Liu X, Sui M. 2016; Self-assembling doxorubicin prodrug forming nanoparticles and effectively reversing drug resistance in vitro and in vivo. Adv Healthc Mater. 5:2517–2527. DOI: 10.1002/adhm.201600345. PMID: 27529558.
Article27. Mi C, Ma J, Wang KS, Zuo HX, Wang Z, Li MY, Piao LX, Xu GH, Li X, Quan ZS, Jin X. 2017; Imperatorin suppresses proliferation and angiogenesis of human colon cancer cell by targeting HIF-1α via the mTOR/p70S6K/4E-BP1 and MAPK pathways. J Ethnopharmacol. 203:27–38. DOI: 10.1016/j.jep.2017.03.033. PMID: 28341244.
Article28. Rigalli JP, Ciriaci N, Arias A, Ceballos MP, Villanueva SS, Luquita MG, Mottino AD, Ghanem CI, Catania VA, Ruiz ML. 2015; Regulation of multidrug resistance proteins by genistein in a hepatocarcinoma cell line: impact on sorafenib cytotoxicity. PLoS One. 10:e0119502. DOI: 10.1371/journal.pone.0119502. PMID: 25781341. PMCID: PMC4364073. PMID: f67cc32f5d164bbe83f394190ac70a9b.
Article29. Sancho R, Márquez N, Gómez-Gonzalo M, Calzado MA, Bettoni G, Coiras MT, Alcamí J, López-Cabrera M, Appendino G, Muñoz E. 2004; Imperatorin inhibits HIV-1 replication through an Sp1-dependent pathway. J Biol Chem. 279:37349–37359. DOI: 10.1074/jbc.M401993200. PMID: 15218031.
Article30. Shylasree TS, Bryant A, Athavale R. 2013; Chemotherapy and/or radiotherapy in combination with surgery for ovarian carcinosarcoma. Cochrane Database Syst Rev. 2013:CD006246. DOI: 10.1002/14651858.CD006246.pub2. PMID: 23450567. PMCID: PMC6756783.
Article31. Simoni D, Rizzi M, Rondanin R, Baruchello R, Marchetti P, Invidiata FP, Labbozzetta M, Poma P, Carina V, Notarbartolo M, Alaimo A, D'Alessandro N. 2008; Antitumor effects of curcumin and structurally beta-diketone modified analogs on multidrug resistant cancer cells. Bioorg Med Chem Lett. 18:845–849. DOI: 10.1016/j.bmcl.2007.11.021. PMID: 18039573.
Article32. Suo A, Qian J, Xu M, Xu W, Zhang Y, Yao Y. 2017; Folate-decorated PEGylated triblock copolymer as a pH/reduction dual-responsive nanovehicle for targeted intracellular co-delivery of doxorubicin and Bcl-2 siRNA. Mater Sci Eng C Mater Biol Appl. 76:659–672. DOI: 10.1016/j.msec.2017.03.124. PMID: 28482576.
Article33. Suo A, Qian J, Zhang Y, Liu R, Xu W, Wang H. 2016; Comb-like amphiphilic polypeptide-based copolymer nanomicelles for co-delivery of doxorubicin and P-gp siRNA into MCF-7 cells. Mater Sci Eng C Mater Biol Appl. 62:564–573. DOI: 10.1016/j.msec.2016.02.007. PMID: 26952460.
Article34. Wu J, Zhang J, Jiang M, Zhang T, Wang Y, Wang Z, Miao Y, Wang Z, Li W. 2018; Comparison between NOD/SCID mice and BALB/c mice for patient-derived tumor xenografts model of non-small-cell lung cancer. Cancer Manag Res. 10:6695–6703. DOI: 10.2147/CMAR.S181272. PMID: 30584364. PMCID: PMC6289205.
Article35. Xiao CQ, Chen R, Lin J, Wang G, Chen Y, Tan ZR, Zhou HH. 2012; Effect of genistein on the activities of cytochrome P450 3A and P-glycoprotein in Chinese healthy participants. Xenobiotica. 42:173–178. DOI: 10.3109/00498254.2011.615954. PMID: 21943317.
Article36. Yuan J, Yin Z, Tan L, Zhu W, Tao K, Wang G, Shi W, Gao J. 2019; Interferon regulatory factor-1 reverses chemoresistance by downregulating the expression of P-glycoprotein in gastric cancer. Cancer Lett. 457:28–39. DOI: 10.1016/j.canlet.2019.05.006. PMID: 31078735.
Article37. Zhang J, Chen Y, Li X, Liang X, Luo X. 2016; The influence of different long-circulating materials on the pharmacokinetics of liposomal vincristine sulfate. Int J Nanomedicine. 11:4187–4197. DOI: 10.2147/IJN.S109547. PMID: 27616886. PMCID: PMC5008646.38. Zhang W, Liu M, Yang L, Huang F, Lan Y, Li H, Wu H, Zhang B, Shi H, Wu X. 2019; P-glycoprotein inhibitor tariquidar potentiates efficacy of astragaloside IV in experimental autoimmune encephalomyelitis mice. Molecules. 24:561. DOI: 10.3390/molecules24030561. PMID: 30717494. PMCID: PMC6384695.
Article39. Zhang S, Yang X, Morris ME. 2004; Combined effects of multiple flavonoids on breast cancer resistance protein (ABCG2)-mediated transport. Pharm Res. 21:1263–1273. DOI: 10.1023/B:PHAM.0000033015.84146.4c. PMID: 15290869.
Article40. Yue Q, Xu Y, Deng X, Wang S, Qiu J, Qian B, Zhang Y. 2021; Circ-PITX1 promotes the progression of non-small cell lung cancer through regulating the miR-1248/CCND2 axis. Onco Targets Ther. 14:1807–1819. DOI: 10.2147/OTT.S286820. PMID: 33727831. PMCID: PMC7955706.
Article41. Lee ACK, Lau PM, Kwan YW, Kong SK. 2021; Mitochondrial fuel dependence on glutamine drives chemo-resistance in the cancer stem cells of hepatocellular carcinoma. Int J Mol Sci. 22:3315. DOI: 10.3390/ijms22073315. PMID: 33805044. PMCID: PMC8036982. PMID: 9147582cd661434a8e0fd39308da65b9.
Article42. Zhao BX, Sun YB, Wang SQ, Duan L, Huo QL, Ren F, Li GF. 2013; Grape seed procyanidin reversal of p-glycoprotein associated multi-drug resistance via down-regulation of NF-κB and MAPK/ERK mediated YB-1 activity in A2780/T cells. PLoS One. 8:e71071. DOI: 10.1371/journal.pone.0071071. PMID: 23967153. PMCID: PMC3744527.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Retrovirus Mediated TNF-α Gene Transfer to Tumor Necrosis Factor(TNF) Sensitive Tumor Cell Lines on Sensitivity to TNF
- Stimulatory Effect of IL-10 on Antitumor Cytolytic Activity of Murine Spleen Cells
- Restoration of Adriamycin and Vincristine Dependent Tumoricidal Activity by Interferon in Mice with Implanted Tumor Cells
- Anti-tumor Effect of the Complex of Acriflavine and Guanosine (AG60)
- Role of HIF-1α in the Responses of Tumors to Radiotherapy and Chemotherapy