Endocrinol Metab.
2022 Apr;37(2):303-311. 10.3803/EnM.2021.1332.
Repeated Low High-Density Lipoprotein Cholesterol and the Risk of Thyroid Cancer: A Nationwide Population- Based Study in Korea
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Yeouido St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Department of Statistics and Actuarial Science, Soongsil University, Seoul, Korea
- KMID: 2529222
- DOI: http://doi.org/10.3803/EnM.2021.1332
Abstract
- Background
High-density lipoprotein cholesterol (HDL-C) plays an important role in the reverse cholesterol transport pathway and prevents atherosclerosis-mediated disease. It has also been suggested that HDL-C may be a protective factor against cancer. However, an inverse correlation between HDL-C and cancer has not been established, and few studies have explored thyroid cancer.
Methods
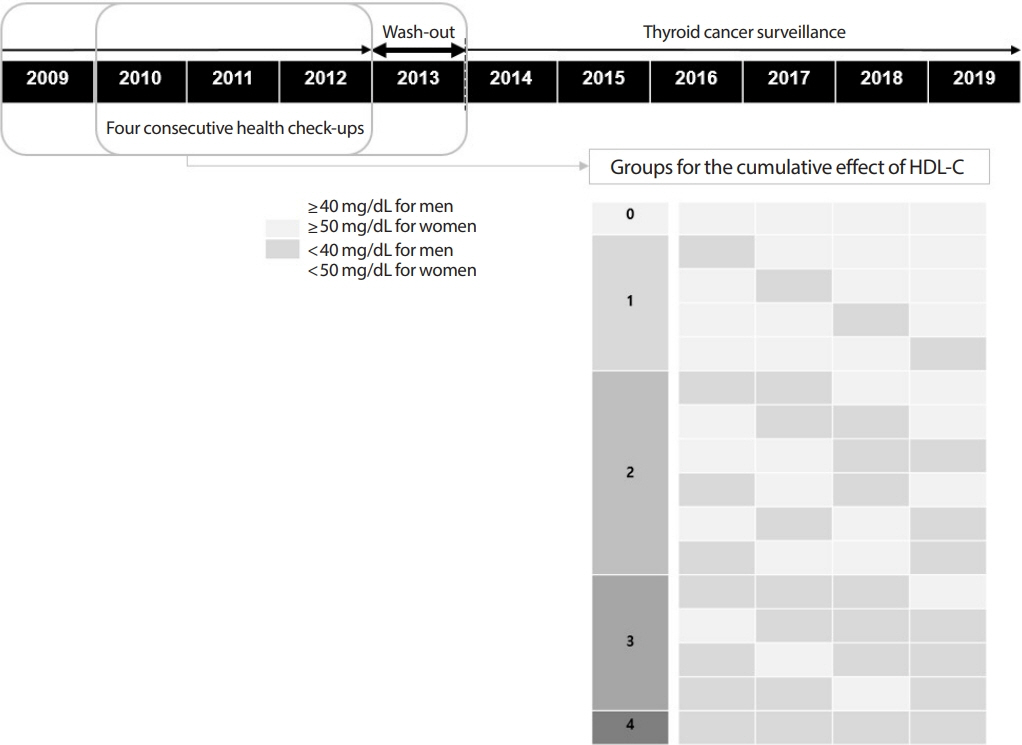
The study participants received health checkups provided by the Korean National Health Insurance Service from 2009 to 2013 and were followed until 2019. Considering the variability of serum HDL-C level, low HDL-C level was analyzed by grouping based on four consecutive health checkups. The data analysis was performed using univariate and multivariate Cox proportional hazard regression models.
Results
A total of 3,134,278 total study participants, thyroid cancer occurred in 16,129. In the crude model, the hazard ratios for the association between repeatedly measured low HDL-C levels and thyroid cancer were 1.243, 1.404, 1.486, and 1.680 (P for trend <0.01), respectively, which were significant even after adjusting for age, sex, lifestyle factors, and metabolic diseases. The subgroup analysis revealed that low HDL-C levels likely had a greater impact on the group of patients with central obesity (P for interaction= 0.062), high blood pressure (P for interaction=0.057), impaired fasting glucose (P for interaction=0.051), and hyperlipidemia (P for interaction=0.126).
Conclusion
Repeatedly measured low HDL-C levels can be considered a risk factor for cancer as well as vascular disease. Low HDL-C levels were associated with the risk of thyroid cancer, and this correlation was stronger in a metabolically unhealthy population.
Figure
Reference
-
1. Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010; 327:46–50.
Article2. Assmann G, Gotto AM Jr. HDL cholesterol and protective factors in atherosclerosis. Circulation. 2004; 109(23 Suppl 1):III8–14.
Article3. McTaggart F, Jones P. Effects of statins on high-density lipoproteins: a potential contribution to cardiovascular benefit. Cardiovasc Drugs Ther. 2008; 22:321–38.
Article4. van Duijnhoven FJ, Bueno-De-Mesquita HB, Calligaro M, Jenab M, Pischon T, Jansen EH, et al. Blood lipid and lipoprotein concentrations and colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition. Gut. 2011; 60:1094–102.5. Ahn J, Lim U, Weinstein SJ, Schatzkin A, Hayes RB, Virtamo J, et al. Prediagnostic total and high-density lipoprotein cholesterol and risk of cancer. Cancer Epidemiol Biomarkers Prev. 2009; 18:2814–21.
Article6. Pedersen KM, Colak Y, Bojesen SE, Nordestgaard BG. Low high-density lipoprotein and increased risk of several cancers: 2 population-based cohort studies including 116,728 individuals. J Hematol Oncol. 2020; 13:129.
Article7. Furberg AS, Veierod MB, Wilsgaard T, Bernstein L, Thune I. Serum high-density lipoprotein cholesterol, metabolic profile, and breast cancer risk. J Natl Cancer Inst. 2004; 96:1152–60.
Article8. Kucharska-Newton AM, Rosamond WD, Schroeder JC, McNeill AM, Coresh J, Folsom AR, et al. HDL-cholesterol and the incidence of lung cancer in the Atherosclerosis Risk in Communities (ARIC) study. Lung Cancer. 2008; 61:292–300.
Article9. Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006; 295:2164–7.
Article10. Kim TY, Kim WG, Kim WB, Shong YK. Current status and future perspectives in differentiated thyroid cancer. Endocrinol Metab (Seoul). 2014; 29:217–25.
Article11. Morris LG, Myssiorek D. Improved detection does not fully explain the rising incidence of well-differentiated thyroid cancer: a population-based analysis. Am J Surg. 2010; 200:454–61.
Article12. Marcello MA, Cunha LL, Batista FA, Ward LS. Obesity and thyroid cancer. Endocr Relat Cancer. 2014; 21:T255–71.
Article13. Xu L, Port M, Landi S, Gemignani F, Cipollini M, Elisei R, et al. Obesity and the risk of papillary thyroid cancer: a pooled analysis of three case-control studies. Thyroid. 2014; 24:966–74.
Article14. Fussey JM, Beaumont RN, Wood AR, Vaidya B, Smith J, Tyrrell J. Does obesity cause thyroid cancer? A Mendelian randomization study. J Clin Endocrinol Metab. 2020; 105:e2398–407.
Article15. Kitahara CM, Platz EA, Freeman LE, Hsing AW, Linet MS, Park Y, et al. Obesity and thyroid cancer risk among U.S. men and women: a pooled analysis of five prospective studies. Cancer Epidemiol Biomarkers Prev. 2011; 20:464–72.
Article16. Kwon H, Han KD, Park CY. Weight change is significantly associated with risk of thyroid cancer: a nationwide population-based cohort study. Sci Rep. 2019; 9:1546.
Article17. Park JH, Choi M, Kim JH, Kim J, Han K, Kim B, et al. Metabolic syndrome and the risk of thyroid cancer: a nationwide population-based cohort study. Thyroid. 2020; 30:1496–504.
Article18. Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort profile: the National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol. 2017; 46:e15.
Article19. Ahn HY, Park YJ. Incidence and clinical characteristics of thyroid cancer in Korea. Korean J Med. 2009; 77:537–42.20. Rossi ED, Pantanowitz L, Hornick JL. A worldwide journey of thyroid cancer incidence centred on tumour histology. Lancet Diabetes Endocrinol. 2021; 9:193–4.
Article21. Ayturk S, Gursoy A, Kut A, Anil C, Nar A, Tutuncu NB. Metabolic syndrome and its components are associated with increased thyroid volume and nodule prevalence in a mildto-moderate iodine-deficient area. Eur J Endocrinol. 2009; 161:599–605.
Article22. Rojeski MT, Gharib H. Nodular thyroid disease. Evaluation and management. N Engl J Med. 1985; 313:428–36.23. Li D, Zhou L, Ma C, Chen W, Zhang Y, Yu S, et al. Comparative analysis of the serum proteome profiles of thyroid cancer: an initial focus on the lipid profile. Oncol Lett. 2019; 18:3349–57.
Article24. Zhao W, Guan J, Horswell R, Li W, Wang Y, Wu X, et al. HDL cholesterol and cancer risk among patients with type 2 diabetes. Diabetes Care. 2014; 37:3196–203.
Article25. Kwon H, Chang Y, Cho A, Ahn J, Park SE, Park CY, et al. Metabolic obesity phenotypes and thyroid cancer risk: a cohort study. Thyroid. 2019; 29:349–58.
Article26. Jafri H, Alsheikh-Ali AA, Karas RH. Baseline and on-treatment high-density lipoprotein cholesterol and the risk of cancer in randomized controlled trials of lipid-altering therapy. J Am Coll Cardiol. 2010; 55:2846–54.
Article27. Barter PJ, Brandrup-Wognsen G, Palmer MK, Nicholls SJ. Effect of statins on HDL-C: a complex process unrelated to changes in LDL-C. Analysis of the VOYAGER Database. J Lipid Res. 2010; 51:1546–53.
Article28. Rashid S, Uffelman KD, Lewis GF. The mechanism of HDL lowering in hypertriglyceridemic, insulin-resistant states. J Diabetes Complications. 2002; 16:24–8.
Article29. Aschebrook-Kilfoy B, Sabra MM, Brenner A, Moore SC, Ron E, Schatzkin A, et al. Diabetes and thyroid cancer risk in the National Institutes of Health-AARP Diet and Health Study. Thyroid. 2011; 21:957–63.
Article30. Fox CS, Pencina MJ, D’Agostino RB, Murabito JM, Seely EW, Pearce EN, et al. Relations of thyroid function to body weight: cross-sectional and longitudinal observations in a community-based sample. Arch Intern Med. 2008; 168:587–92.
Article31. Iacobellis G, Ribaudo MC, Zappaterreno A, Iannucci CV, Leonetti F. Relationship of thyroid function with body mass index, leptin, insulin sensitivity and adiponectin in euthyroid obese women. Clin Endocrinol (Oxf). 2005; 62:487–91.
Article32. Kim HJ, Park SJ, Park HK, Byun DW, Suh K, Yoo MH. Thyroid autoimmunity and metabolic syndrome: a nationwide population-based study. Eur J Endocrinol. 2021; 185:707–15.
Article33. Bjoro T, Holmen J, Kruger O, Midthjell K, Hunstad K, Schreiner T, et al. Prevalence of thyroid disease, thyroid dysfunction and thyroid peroxidase antibodies in a large, unselected population. The Health Study of Nord-Trondelag (HUNT). Eur J Endocrinol. 2000; 143:639–47.
Article34. Lacroix L, Pourcher T, Magnon C, Bellon N, Talbot M, Intaraphairot T, et al. Expression of the apical iodide transporter in human thyroid tissues: a comparison study with other iodide transporters. J Clin Endocrinol Metab. 2004; 89:1423–8.
Article35. Ichikawa Y, Saito E, Abe Y, Homma M, Muraki T. Presence of TSH receptor in thyroid neoplasms. J Clin Endocrinol Metab. 1976; 42:395–8.
Article36. Fiore E, Rago T, Provenzale MA, Scutari M, Ugolini C, Basolo F, et al. Lower levels of TSH are associated with a lower risk of papillary thyroid cancer in patients with thyroid nodular disease: thyroid autonomy may play a protective role. Endocr Relat Cancer. 2009; 16:1251–60.
Article37. Duntas LH, Brenta G. A renewed focus on the association between thyroid hormones and lipid metabolism. Front Endocrinol (Lausanne). 2018; 9:511.
Article38. Willard DL, Leung AM, Pearce EN. Thyroid function testing in patients with newly diagnosed hyperlipidemia. JAMA Intern Med. 2014; 174:287–9.
Article39. Vekic J, Kotur-Stevuljevic J, Jelic-Ivanovic Z, Spasic S, Spasojevic- Kalimanovska V, Topic A, et al. Association of oxidative stress and PON1 with LDL and HDL particle size in middle-aged subjects. Eur J Clin Invest. 2007; 37:715–23.
Article40. Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, et al. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 2010; 328:1689–93.
Article41. Morin EE, Li XA, Schwendeman A. HDL in endocrine carcinomas: biomarker, drug carrier, and potential therapeutic. Front Endocrinol (Lausanne). 2018; 9:715.
Article42. Han MR, Ju DL, Park YJ, Paik HY, Song Y. An iodine database for common Korean foods and the association between iodine intake and thyroid disease in Korean adults. Int J Thyroidol. 2015; 8:170–82.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- High Density Lipoprotein Cholesterol Comes of Age
- Changes in the Serum Level of High Density Lipoprotein-cholesterol after Smoking Cessation among Adult Men
- Predictors of Serum Low-Density Lipoprotein Cholesterol Level in Postmenopausal Women
- Hypolipidemic Effects and Safety of Lovastatin in Patients with Primary Hypercholesterolemia
- Discrepant Effect of High-Density Lipoprotein Cholesterol on the Hematologic Malignancy Risk: A Nationwide Cohort Study