Endocrinol Metab.
2022 Apr;37(2):195-207. 10.3803/EnM.2022.1404.
A Study on Methodologies of Drug Repositioning Using Biomedical Big Data: A Focus on Diabetes Mellitus
- Affiliations
-
- 1Department of Biomedical Informatics, Konyang University College of Medicine, Daejeon, Korea
- 2Health Care Data Science Center, Konyang University Hospital, Daejeon, Korea
- 3Department of Medical Informatics, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 4Division of Endocrinology and Metabolism, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2529211
- DOI: http://doi.org/10.3803/EnM.2022.1404
Abstract
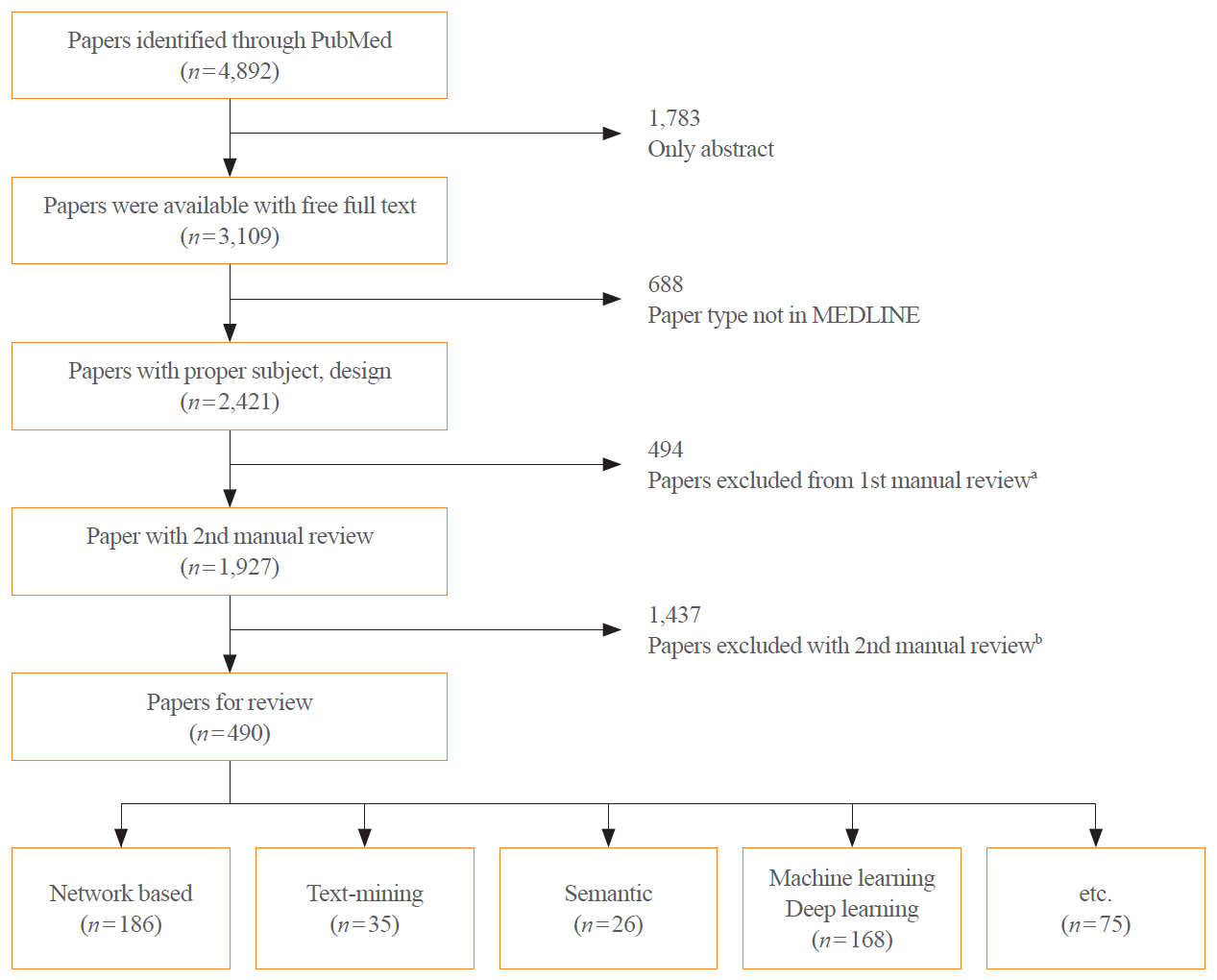
- Drug repositioning is a strategy for identifying new applications of an existing drug that has been previously proven to be safe. Based on several examples of drug repositioning, we aimed to determine the methodologies and relevant steps associated with drug repositioning that should be pursued in the future. Reports on drug repositioning, retrieved from PubMed from January 2011 to December 2020, were classified based on an analysis of the methodology and reviewed by experts. Among various drug repositioning methods, the network-based approach was the most common (38.0%, 186/490 cases), followed by machine learning/deep learningbased (34.3%, 168/490 cases), text mining-based (7.1%, 35/490 cases), semantic-based (5.3%, 26/490 cases), and others (15.3%, 75/490 cases). Although drug repositioning offers several advantages, its implementation is curtailed by the need for prior, conclusive clinical proof. This approach requires the construction of various databases, and a deep understanding of the process underlying repositioning is quintessential. An in-depth understanding of drug repositioning could reduce the time, cost, and risks inherent to early drug development, providing reliable scientific evidence. Furthermore, regarding patient safety, drug repurposing might allow the discovery of new relationships between drugs and diseases.
Figure
Reference
-
1. Chakravarty K, Antontsev VG, Khotimchenko M, Gupta N, Jagarapu A, Bundey Y, et al. Accelerated repurposing and drug development of pulmonary hypertension therapies for COVID-19 treatment using an AI-integrated biosimulation platform. Molecules. 2021; 26:1912.
Article2. Spinelli A, Pellino G. COVID-19 pandemic: perspectives on an unfolding crisis. Br J Surg. 2020; 107:785–7.
Article3. Jarada TN, Rokne JG, Alhajj R. A review of computational drug repositioning: strategies, approaches, opportunities, challenges, and directions. J Cheminform. 2020; 12:46.
Article4. Yeu Y, Yoon Y, Park S. Protein localization vector propagation: a method for improving the accuracy of drug repositioning. Mol Biosyst. 2015; 11:2096–102.
Article5. Saberian N, Peyvandipour A, Donato M, Ansari S, Draghici S. A new computational drug repurposing method using established disease-drug pair knowledge. Bioinformatics. 2019; 35:3672–8.
Article6. Mohapatra S, Nath P, Chatterjee M, Das N, Kalita D, Roy P, et al. Repurposing therapeutics for COVID-19: rapid prediction of commercially available drugs through machine learning and docking. PLoS One. 2020; 15:e0241543.
Article7. Mickael ME, Pajares M, Enache I, Manda G, Cuadrado A. Nrf2 drug repurposing using a question-answer artificial intelligence system. bioRxiv. 2019; Apr. 4. [Preprint]. https://www.biorxiv.org/content/10.1101/594622v1.
Article8. Ghofrani HA, Osterloh IH, Grimminger F. Sildenafil: from angina to erectile dysfunction to pulmonary hypertension and beyond. Nat Rev Drug Discov. 2006; 5:689–702.
Article9. Ekman P. Finasteride in the treatment of benign prostatic hypertrophy: an update. New indications for finasteride therapy. Scand J Urol Nephrol Suppl. 1999; 203:15–20.
Article10. Sanchez-Garcia A, Simental-Mendia M, Millan-Alanis JM, Simental-Mendia LE. Effect of sodium-glucose co-transporter 2 inhibitors on lipid profile: a systematic review and meta-analysis of 48 randomized controlled trials. Pharmacol Res. 2020; 160:105068.11. Filippatos TD, Tsimihodimos V, Elisaf MS. Mechanisms of blood pressure reduction with sodium-glucose co-transporter 2 (SGLT2) inhibitors. Expert Opin Pharmacother. 2016; 17:1581–3.
Article12. Ryan D, Acosta A. GLP-1 receptor agonists: nonglycemic clinical effects in weight loss and beyond. Obesity (Silver Spring). 2015; 23:1119–29.13. Blommel ML, Blommel AL. Pregabalin: an antiepileptic agent useful for neuropathic pain. Am J Health Syst Pharm. 2007; 64:1475–82.
Article14. Paranjpe MD, Taubes A, Sirota M. Insights into computational drug repurposing for neurodegenerative disease. Trends Pharmacol Sci. 2019; 40:565–76.
Article15. Xue H, Li J, Xie H, Wang Y. Review of drug repositioning approaches and resources. Int J Biol Sci. 2018; 14:1232–44.
Article16. Keiser MJ, Setola V, Irwin JJ, Laggner C, Abbas AI, Hufeisen SJ, et al. Predicting new molecular targets for known drugs. Nature. 2009; 462:175–81.
Article17. Iorio F, Bosotti R, Scacheri E, Belcastro V, Mithbaokar P, Ferriero R, et al. Discovery of drug mode of action and drug repositioning from transcriptional responses. Proc Natl Acad Sci U S A. 2010; 107:14621–6.
Article18. Tian Z, Teng Z, Cheng S, Guo M. Computational drug repositioning using meta-path-based semantic network analysis. BMC Syst Biol. 2018; 12(Suppl 9):134.
Article19. Wu Z, Wang Y, Chen L. Network-based drug repositioning. Mol Biosyst. 2013; 9:1268–81.
Article20. Wu C, Gudivada RC, Aronow BJ, Jegga AG. Computational drug repositioning through heterogeneous network clustering. BMC Syst Biol. 2013; 7 Suppl 5:S6.
Article21. Kanehisa M, Sato Y, Kawashima M, Furumichi M, Tanabe M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016; 44:D457–62.
Article22. Kyoto Encyclopedia of Genes and Genomes. KEGG: Kyoto Encyclopedia of genes and genomes [Internet]. Kyoto: Kanehisa Laboratories;2021. [cited 2022 Mar 26]. Available from: https://www.genome.jp/kegg/.23. Cheng F, Desai RJ, Handy DE, Wang R, Schneeweiss S, Barabasi AL, et al. Network-based approach to prediction and population-based validation of in silico drug repurposing. Nat Commun. 2018; 9:2691.
Article24. Emig D, Ivliev A, Pustovalova O, Lancashire L, Bureeva S, Nikolsky Y, et al. Drug target prediction and repositioning using an integrated network-based approach. PLoS One. 2013; 8:e60618.
Article25. Goldstein JA, Bastarache LA, Denny JC, Roden DM, Pulley JM, Aronoff DM. Calcium channel blockers as drug repurposing candidates for gestational diabetes: mining large scale genomic and electronic health records data to repurpose medications. Pharmacol Res. 2018; 130:44–51.
Article26. Rotermund C, Machetanz G, Fitzgerald JC. The therapeutic potential of metformin in neurodegenerative diseases. Front Endocrinol (Lausanne). 2018; 9:400.
Article27. Datanet. Development of new drugs using AI (3): Using text mining to streamline new drug development [Internet]. Seoul: Network TIMES;2021. [updated 2021 Jun 3; cited 2022 Mar 26]. Available from: https://www.datanet.co.kr/news/articleView.html?idxno=160227.28. Fleuren WW, Alkema W. Application of text mining in the biomedical domain. Methods. 2015; 74:97–106.
Article29. Kostoff RN, Briggs MB, Shores DR. Treatment repurposing for inflammatory bowel disease using literature-related discovery and innovation. World J Gastroenterol. 2020; 26:4889–99.
Article30. Zhang M, Luo H, Xi Z, Rogaeva E. Drug repositioning for diabetes based on ‘omics’ data mining. PLoS One. 2015; 10:e0126082.
Article31. Zhang L, Hu J, Xu Q, Li F, Rao G, Tao C. A semantic relationship mining method among disorders, genes, and drugs from different biomedical datasets. BMC Med Inform Decis Mak. 2020; 20(Suppl 4):283.
Article32. Pijl H, Ohashi S, Matsuda M, Miyazaki Y, Mahankali A, Kumar V, et al. Bromocriptine: a novel approach to the treatment of type 2 diabetes. Diabetes Care. 2000; 23:1154–61.
Article33. Napolitano F, Zhao Y, Moreira VM, Tagliaferri R, Kere J, D’Amato M, et al. Drug repositioning: a machine-learning approach through data integration. J Cheminform. 2013; 5:30.
Article34. Menden MP, Iorio F, Garnett M, McDermott U, Benes CH, Ballester PJ, et al. Machine learning prediction of cancer cell sensitivity to drugs based on genomic and chemical properties. PLoS One. 2013; 8:e61318.
Article35. Koren G, Nordon G, Radinsky K, Shalev V. Identification of repurposable drugs with beneficial effects on glucose control in type 2 diabetes using machine learning. Pharmacol Res Perspect. 2019; 7:e00529.
Article36. Ng L, Foo DC, Wong CK, Man AT, Lo OS, Law WL. Repurposing DPP-4 inhibitors for colorectal cancer: a retrospective and single center study. Cancers (Basel). 2021; 13:3588.
Article37. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020; 323:1824–36.38. Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020; 14:72–3.
Article39. Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020; 56:105949.
Article40. Baruah P, Das A, Paul D, Chakrabarty S, Aguan K, Mitra S. Sulfonylurea class of antidiabetic drugs inhibit acetylcholinesterase activity: unexplored auxiliary pharmacological benefit toward Alzheimer’s disease. ACS Pharmacol Transl Sci. 2021; 4:193–205.
Article41. Xu H, Aldrich MC, Chen Q, Liu H, Peterson NB, Dai Q, et al. Validating drug repurposing signals using electronic health records: a case study of metformin associated with reduced cancer mortality. J Am Med Inform Assoc. 2015; 22:179–91.
Article42. Sharma S, Nozohouri S, Vaidya B, Abbruscato T. Repurposing metformin to treat age-related neurodegenerative disorders and ischemic stroke. Life Sci. 2021; 274:119343.
Article43. Moulton CD, Hopkins C, Ismail K, Stahl D. Repositioning of diabetes treatments for depressive symptoms: a systematic review and meta-analysis of clinical trials. Psychoneuroendocrinology. 2018; 94:91–103.
Article44. Wahlqvist ML, Lee MS, Chuang SY, Hsu CC, Tsai HN, Yu SH, et al. Increased risk of affective disorders in type 2 diabetes is minimized by sulfonylurea and metformin combination: a population-based cohort study. BMC Med. 2012; 10:150.
Article45. Rao PPN, Pham AT, Shakeri A, El Shatshat A, Zhao Y, Karuturi RC, et al. Drug repurposing: dipeptidyl peptidase IV (DPP4) inhibitors as potential agents to treat SARS-CoV-2 (2019-nCoV) infection. Pharmaceuticals (Basel). 2021; 14:44.
Article46. Bonora BM, Raschi E, Avogaro A, Fadini GP. SGLT-2 inhibitors and atrial fibrillation in the Food and Drug Administration adverse event reporting system. Cardiovasc Diabetol. 2021; 20:39.47. Pintana H, Apaijai N, Chattipakorn N, Chattipakorn SC. DPP-4 inhibitors improve cognition and brain mitochondrial function of insulin-resistant rats. J Endocrinol. 2013; 218:1–11.
Article48. Markaki I, Winther K, Catrina SB, Svenningsson P. Repurposing GLP1 agonists for neurodegenerative diseases. Int Rev Neurobiol. 2020; 155:91–112.
Article49. Daghlas I, Karhunen V, Ray D, Zuber V, Burgess S, Tsao PS, et al. Genetic evidence for repurposing of GLP1R (glucagon-like peptide-1 receptor) agonists to prevent heart failure. J Am Heart Assoc. 2021; 10:e020331.
Article50. Foltynie T, Athauda D. Repurposing anti-diabetic drugs for the treatment of Parkinson’s disease: rationale and clinical experience. Prog Brain Res. 2020; 252:493–523.
Article51. Rameshrad M, Razavi BM, Lalau JD, De Broe ME, Hosseinzadeh H. An overview of glucagon-like peptide-1 receptor agonists for the treatment of metabolic syndrome: a drug repositioning. Iran J Basic Med Sci. 2020; 23:556–68.52. Levy P, Jellinger PS. The potential role of colesevelam in the management of prediabetes and type 2 diabetes mellitus. Postgrad Med. 2010; 122(3 Suppl):1–8.53. Magkou D, Tziomalos K. Antidiabetic treatment, stroke severity and outcome. World J Diabetes. 2014; 5:84–8.
Article54. Kobayashi Y, Banno K, Kunitomi H, Tominaga E, Aoki D. Current state and outlook for drug repositioning anticipated in the field of ovarian cancer. J Gynecol Oncol. 2019; 30:e10.
Article55. Xu H, Li J, Jiang X, Chen Q. Electronic health records for drug repurposing: current status, challenges, and future directions. Clin Pharmacol Ther. 2020; 107:712–4.
Article56. Oprea TI, Bauman JE, Bologa CG, Buranda T, Chigaev A, Edwards BS, et al. Drug repurposing from an academic perspective. Drug Discov Today Ther Strateg. 2011; 8:61–9.
Article57. ChEMBL. ChEMBL database [Internet]. Hinxton: The European Bioinformatics Institute (EMBL-EBI);2018. [cited 2022 Mar 26]. Available from: https://www.ebi.ac.uk/chembl/.58. Broad Institute. ChemBank [Internet]. Cambridge: Broad Institute;[cited 2022 Mar 26]. Available from: http://chembank. broad.harvard.edu/.59. Seiler KP, George GA, Happ MP, Bodycombe NE, Carrinski HA, Norton S, et al. ChemBank: a small-molecule screening and cheminformatics resource database. Nucleic Acids Res. 2008; 36:D351–9.
Article60. U.S. National Library of Medicine. clinicaltrials.gov [Internet]. Bethesda: National Library of Medicine;2021. [cited 2022 Mar 26]. Available from: https://clinicaltrials.gov/.61. DailyMed. Dailymed overview [Internet]. Bethesda: National Library of Medicine;2021. [cited 2022 Mar 26]. Available from: https://dailymed.nlm.nih.gov/dailymed/about-dailymed.cfm.62. DrugBank. DrugBank online: database for drug and drug target info [Internet]. Alberta: DrugBank;2017. [cited 2022 Mar 26]. Available from: https://go.drugbank.com/.63. Xu R, Wang Q. Large-scale combining signals from both biomedical literature and the FDA Adverse Event Reporting System (FAERS) to improve post-marketing drug safety signal detection. BMC Bioinformatics. 2014; 15:17.
Article64. U.S. Food and Drug Administration. FDA adverse event reporting System (FAERS) Public Dashboard [Internet]. Silver Spring: FDA;2021. [cited 2022 Mar 26]. Available from: https://www.fda.gov/drugs/questions-and-answers-fdasadverse-event-reporting-system-faers/fda-adverse-eventreporting-system-faers-public-dashboard.65. Gene ontology. The gene ontology resource [Internet]. Bar Harbor: The Gene Ontology Consortium;2021. [cited 2022 Mar 26]. Available from: http://www.geneontology.org/.66. MedHelp. Be your healthiest [Internet]. San Francisco: MedHelp;2021. [cited 2022 Mar 26]. Available from: https://www.medhelp.org/.67. National Library of Medicine. Medline: overview [Internet]. Bethesda: National Library of Medicine;2021. [cited 2022 Mar 26]. Available from: https://www.nlm.nih.gov/medline/medline_overview.html.68. MedlinePlus. Health information from the National library of medicine [Internet]. Bethesda: National Library of Medicine;2021. [cited 2022 Mar 26]. Available from: https://medlineplus.gov/.69. Lowe HJ, Barnett GO. Understanding and using the medical subject headings (MeSH) vocabulary to perform literature searches. JAMA. 1994; 271:1103–8.
Article70. Hamosh A, Scott AF, Amberger JS, Bocchini CA, McKusick VA. Online Mendelian Inheritance in Man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Res. 2005; 33:D514–7.
Article71. PharmGKB. Search pharmGKB [Internet]. Stanford: PharmGKB;2021. [cited 2022 Mar 26]. Available from: https://www.pharmgkb.org/.72. Thorn CF, Klein TE, Altman RB. PharmGKB: the Pharmacogenomics Knowledge Base. Methods Mol Biol. 2013; 1015:311–20.
Article73. PreMedKB. Precision Medicine Knowledge Base [Internet]. Shanghai: PreMedKB;2018. [cited 2022 Mar 26]. Available from: http://www.fudan-pgx.org/premedkb/index.html#/home.74. PubChem. Explore chemistry [Internet]. Bethesda: National Library of Medicine;2021. [cited 2022 Mar 26]. Available from: https://pubchem.ncbi.nlm.nih.gov/.75. National Library of Medicine. PubMed [Internet]. Bethesda: National Library of Medicine;2021. [cited 2022 Mar 26]. Available from: https://pubmed.ncbi.nlm.nih.gov/.76. Malas TB, Vlietstra WJ, Kudrin R, Starikov S, Charrout M, Roos M, et al. Drug prioritization using the semantic properties of a knowledge graph. Sci Rep. 2019; 9:6281.
Article77. Rindflesch TC, Kilicoglu H, Fiszman M, Rosemblat G, Shin D. Semantic medline: an advanced information management application for biomedicine. Inf Serv Use. 2011; 31:15–21.
Article78. National Library of Medicine. Access to SemRep/SemMedDB/SKR Resources [Internet]. Bethesda: National Library of Medicine;2021. [cited 2022 Mar 26]. Available from: https://skr3.nlm.nih.gov/SemMedDB/.79. Kuhn M, Campillos M, Letunic I, Jensen LJ, Bork P. A side effect resource to capture phenotypic effects of drugs. Mol Syst Biol. 2010; 6:343.
Article80. SIDER 4.1. SIDER 4.1 Side effect resource [Internet]. SIDER 4.;2015; [cited 2022 Mar 26]. Available from: http://sideeffects.embl.de/.81. Yao L, Zhang Y, Li Y, Sanseau P, Agarwal P. Electronic health records: implications for drug discovery. Drug Discov Today. 2011; 16:594–9.
Article82. Bate A, Juniper J, Lawton AM, Thwaites RM. Designing and incorporating a real world data approach to international drug development and use: what the UK offers. Drug Discov Today. 2016; 21:400–5.
Article83. Kim HS, Lee S, Kim JH. Real-world evidence versus randomized controlled trial: clinical research based on electronic medical records. J Korean Med Sci. 2018; 33:e213.
Article84. Lee S, Kim HS. Prospect of artificial intelligence based on electronic medical record. J Lipid Atheroscler. 2021; 10:282–90.
Article85. Ozery-Flato M, Goldschmidt Y, Shaham O, Ravid S, Yanover C. Framework for identifying drug repurposing candidates from observational healthcare data. JAMIA Open. 2020; 3:536–44.
Article86. Kim DH, Lee JE, Kim YG, Lee Y, Seo DW, Lee KH, et al. High-throughput algorithm for discovering new drug indications by utilizing large-scale electronic medical record data. Clin Pharmacol Ther. 2020; 108:1299–307.
Article87. Kim HS, Kim DJ, Yoon KH. Medical big data is not yet available: why we need realism rather than exaggeration. Endocrinol Metab (Seoul). 2019; 34:349–54.
Article88. Kim HS, Kim JH. Proceed with caution when using real world data and real world evidence. J Korean Med Sci. 2019; 34:e28.
Article89. Shin SY, Kim HS. Data pseudonymization in a range that does not affect data quality: correlation with the degree of participation of clinicians. J Korean Med Sci. 2021; 36:e299.
Article90. Nabirotchkin S, Peluffo AE, Rinaudo P, Yu J, Hajj R, Cohen D. Next-generation drug repurposing using human genetics and network biology. Curr Opin Pharmacol. 2020; 51:78–92.
Article91. Denny JC, Ritchie MD, Basford MA, Pulley JM, Bastarache L, Brown-Gentry K, et al. PheWAS: demonstrating the feasibility of a phenome-wide scan to discover gene-disease associations. Bioinformatics. 2010; 26:1205–10.
Article92. Whirl-Carrillo M, McDonagh EM, Hebert JM, Gong L, Sangkuhl K, Thorn CF, et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther. 2012; 92:414–7.
Article93. Yang L, Agarwal P. Systematic drug repositioning based on clinical side-effects. PLoS One. 2011; 6:e28025.
Article94. Lotfi Shahreza M, Ghadiri N, Mousavi SR, Varshosaz J, Green JR. A review of network-based approaches to drug repositioning. Brief Bioinform. 2018; 19:878–892.
Article95. Chen B, Ma L, Paik H, Sirota M, Wei W, Chua MS, et al. Reversal of cancer gene expression correlates with drug efficacy and reveals therapeutic targets. Nat Commun. 2017; 8:16022.
Article96. Paik H, Chen B, Sirota M, Hadley D, Butte AJ. Integrating clinical phenotype and gene expression data to prioritize novel drug uses. CPT Pharmacometrics Syst Pharmacol. 2016; 5:599–607.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Drug Repositioning Using Temporal Trajectories of Accompanying Comorbidities in Diabetes Mellitus
- Repositioned Drugs for Inflammatory Diseases such as Sepsis, Asthma, and Atopic Dermatitis
- An update on the drug therapy of diabetes mellitus
- The Link Between Sleep and Diabetes Mellitus: A Literature Review
- Sleep Disorders and Diabetes