Cancer Res Treat.
2022 Apr;54(2):563-571. 10.4143/crt.2021.178.
Vincristine, Irinotecan, and Temozolomide as a Salvage Regimen for Relapsed or Refractory Sarcoma in Children and Young Adults
- Affiliations
-
- 1Department of Pediatrics, Samsung Medical Center, Seoul, Korea
- 2Center for Pediatric Oncology, National Cancer Center, Goyang, Korea
- 3Department of Pathology, National Cancer Center, Goyang, Korea
- 4Orthopaedic Oncology Clinic, National Cancer Center, Goyang, Korea
- 5Center for Lung Cancer, Research Institute and Hospital, National Cancer Center, Goyang, Korea
- KMID: 2528225
- DOI: http://doi.org/10.4143/crt.2021.178
Abstract
- Purpose
No standard salvage regimen is available for relapsed or refractory sarcoma. We investigated the efficacy and toxicity of the vincristine, irinotecan, and temozolomide combination (VIT) for relapsed or refractory sarcomas of variable histology in children and young adults.
Materials and Methods
We retrospectively reviewed data from the relapsed or refractory sarcoma patients who were treated with VIT. The VIT protocol was given every 3 weeks as follows: vincristine, 1.5 mg/m2 intravenously on day 1, irinotecan, 50 mg/m2/day intravenously on days 1-5, and temozolomide, 100 mg/m2/day orally on days 1-5.
Results
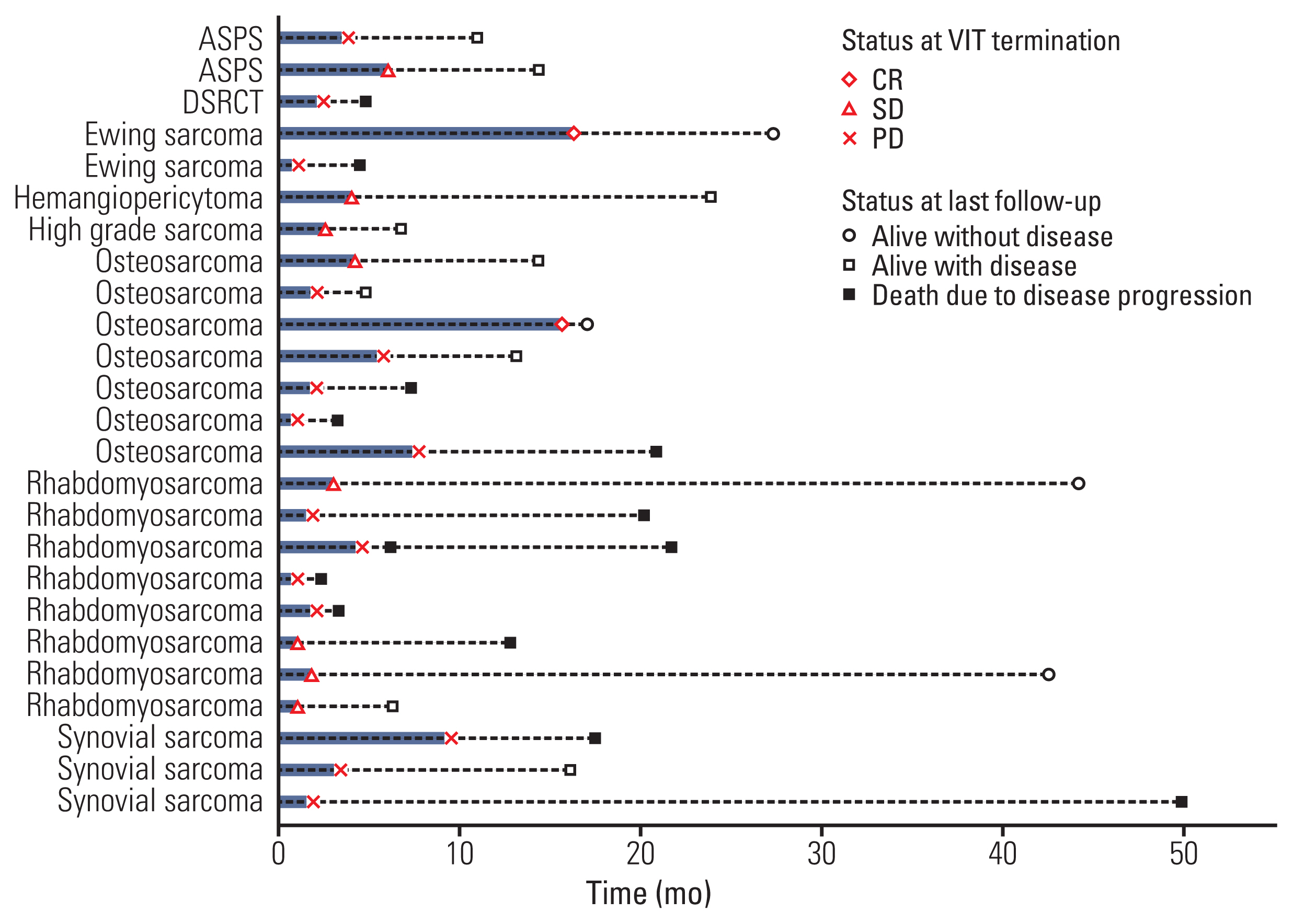
A total of 26 patients (12 males) with various sarcoma histology were included in the study. Most common diagnosis was rhabdomyosarcoma (n=8) followed by osteosarcoma (n=7). Median age at the start of VIT was 18.5 years (range, 2.0 to 39.9). VIT was delivered as 2nd to 7th line of treatment, with 4th line most common (9/26, 34.6%). Median number of VIT courses given was 3 (range, 1 to 18). Of the 25 evaluable patients, there was two partial response (PR) and 11 stable disease (SD) with an overall control rate (complete remission+PR+SD) of 52%. PR was seen in one (50%) of the two evaluable patients with Ewing sarcoma and one (14.3%) of the seven patients with osteosarcoma. Overall survival and progression-free survival rates were 79.3% and 33.9% at 1 year, and 45.5% and 25.4% at 2 years, respectively. There was no treatment-related mortality.
Conclusion
The VIT regimen was effective and relatively safe in our cohort of sarcoma patients.
Keyword
Figure
Reference
-
References
1. Linabery AM, Ross JA. Trends in childhood cancer incidence in the U.S. (1992–2004). Cancer. 2008; 112:416–32.
Article2. Spunt SL, Pappo AS. Childhood nonrhabdomyosarcoma soft tissue sarcomas are not adult-type tumors. J Clin Oncol. 2006; 24:1958–9.
Article3. Ou JY, Spraker-Perlman H, Dietz AC, Smits-Seemann RR, Kaul S, Kirchhoff AC. Conditional survival of pediatric, adolescent, and young adult soft tissue sarcoma and bone tumor patients. Cancer Epidemiol. 2017; 50:150–7.
Article4. Park HJ, Moon EK, Yoon JY, Oh CM, Jung KW, Park BK, et al. Incidence and survival of childhood cancer in Korea. Cancer Res Treat. 2016; 48:869–82.
Article5. Van Winkle P, Angiolillo A, Krailo M, Cheung YK, Anderson B, Davenport V, et al. Ifosfamide, carboplatin, and etoposide (ICE) reinduction chemotherapy in a large cohort of children and adolescents with recurrent/refractory sarcoma: the Children’s Cancer Group (CCG) experience. Pediatr Blood Cancer. 2005; 44:338–47.
Article6. Saylors RL 3rd, Stine KC, Sullivan J, Kepner JL, Wall DA, Bernstein ML, et al. Cyclophosphamide plus topotecan in children with recurrent or refractory solid tumors: a Pediatric Oncology Group phase II study. J Clin Oncol. 2001; 19:3463–9.
Article7. Rapkin L, Qayed M, Brill P, Martin M, Clark D, George BA, et al. Gemcitabine and docetaxel (GEMDOX) for the treatment of relapsed and refractory pediatric sarcomas. Pediatr Blood Cancer. 2012; 59:854–8.
Article8. Buyukkapu Bay S, Kebudi R, Gorgun O, Zulfikar B, Darendeliler E, Cakir FB. Vincristine, irinotecan, and temozolomide treatment for refractory/relapsed pediatric solid tumors: a single center experience. J Oncol Pharm Pract. 2019; 25:1343–8.
Article9. Kurucu N, Sari N, Ilhan IE. Irinotecan and temozolamide treatment for relapsed Ewing sarcoma: a single-center experience and review of the literature. Pediatr Hematol Oncol. 2015; 32:50–9.
Article10. Raciborska A, Bilska K, Drabko K, Chaber R, Pogorzala M, Wyrobek E, et al. Vincristine, irinotecan, and temozolomide in patients with relapsed and refractory Ewing sarcoma. Pediatr Blood Cancer. 2013; 60:1621–5.
Article11. Wagner L. Camptothecin-based regimens for treatment of ewing sarcoma: past studies and future directions. Sarcoma. 2011; 2011:957957.12. Wagner LM, McAllister N, Goldsby RE, Rausen AR, McNall-Knapp RY, McCarville MB, et al. Temozolomide and intravenous irinotecan for treatment of advanced Ewing sarcoma. Pediatr Blood Cancer. 2007; 48:132–9.
Article13. Pappo AS, Lyden E, Breitfeld P, Donaldson SS, Wiener E, Parham D, et al. Two consecutive phase II window trials of irinotecan alone or in combination with vincristine for the treatment of metastatic rhabdomyosarcoma: the Children’s Oncology Group. J Clin Oncol. 2007; 25:362–9.
Article14. Furman WL, Crews KR, Billups C, Wu J, Gajjar AJ, Daw NC, et al. Cefixime allows greater dose escalation of oral irinotecan: a phase I study in pediatric patients with refractory solid tumors. J Clin Oncol. 2006; 24:563–70.
Article15. Nishino M, Jagannathan JP, Ramaiya NH, Van den Abbeele AD. Revised RECIST guideline version 1.1: What oncologists want to know and what radiologists need to know. AJR Am J Roentgenol. 2010; 195:281–9.
Article16. Setty BA, Stanek JR, Mascarenhas L, Miller A, Bagatell R, Okcu F, et al. VIncristine, irinotecan, and temozolomide in children and adolescents with relapsed rhabdomyosarcoma. Pediatr Blood Cancer. 2018; 65:e26728.
Article17. Palmerini E, Jones RL, Setola E, Picci P, Marchesi E, Luksch R, et al. Irinotecan and temozolomide in recurrent Ewing sarcoma: an analysis in 51 adult and pediatric patients. Acta Oncol. 2018; 57:958–64.
Article18. Casey DA, Wexler LH, Merchant MS, Chou AJ, Merola PR, Price AP, et al. Irinotecan and temozolomide for Ewing sarcoma: the Memorial Sloan-Kettering experience. Pediatr Blood Cancer. 2009; 53:1029–34.
Article19. McNall-Knapp RY, Williams CN, Reeves EN, Heideman RL, Meyer WH. Extended phase I evaluation of vincristine, irinotecan, temozolomide, and antibiotic in children with refractory solid tumors. Pediatr Blood Cancer. 2010; 54:909–15.
Article20. Wagner LM, Perentesis JP, Reid JM, Ames MM, Safgren SL, Nelson MD Jr, et al. Phase I trial of two schedules of vincristine, oral irinotecan, and temozolomide (VOIT) for children with relapsed or refractory solid tumors: a Children’s Oncology Group phase I consortium study. Pediatr Blood Cancer. 2010; 54:538–45.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Efficacy of Ifosfamide-Based Regimen in Refractory of Relapsed Ovarian Cancer
- Salvage treatment of relapsed/refractory LCH
- ESHAP Salvage Therapy for Relapsed or Refractory Non-Hodgkin's Lymphoma
- Efficacy and Safety of Melphalan, Cyclophosphamide and Dexamethasone (MCD) as a Salvage Treatment for Patients with Relapsed/Refractory Multiple Myeloma
- Bendamustine in combination with ifosfamide, etoposide, and vinorelbine (VIBE) is an effective salvage regimen for heavily pre-treated patients with relapsed or refractory Hodgkin lymphoma: a single-center experience