Blood Res.
2022 Mar;57(1):6-12. 10.5045/br.2021.2021151.
Convalescent plasma in COVID-19: renewed focus on the timing and effectiveness of an old therapy
- Affiliations
-
- 1Department of Clinical and Chemical Pathology, Faculty of Medicine, Suez Canal University, Ismailia, Egypt
- KMID: 2527491
- DOI: http://doi.org/10.5045/br.2021.2021151
Abstract
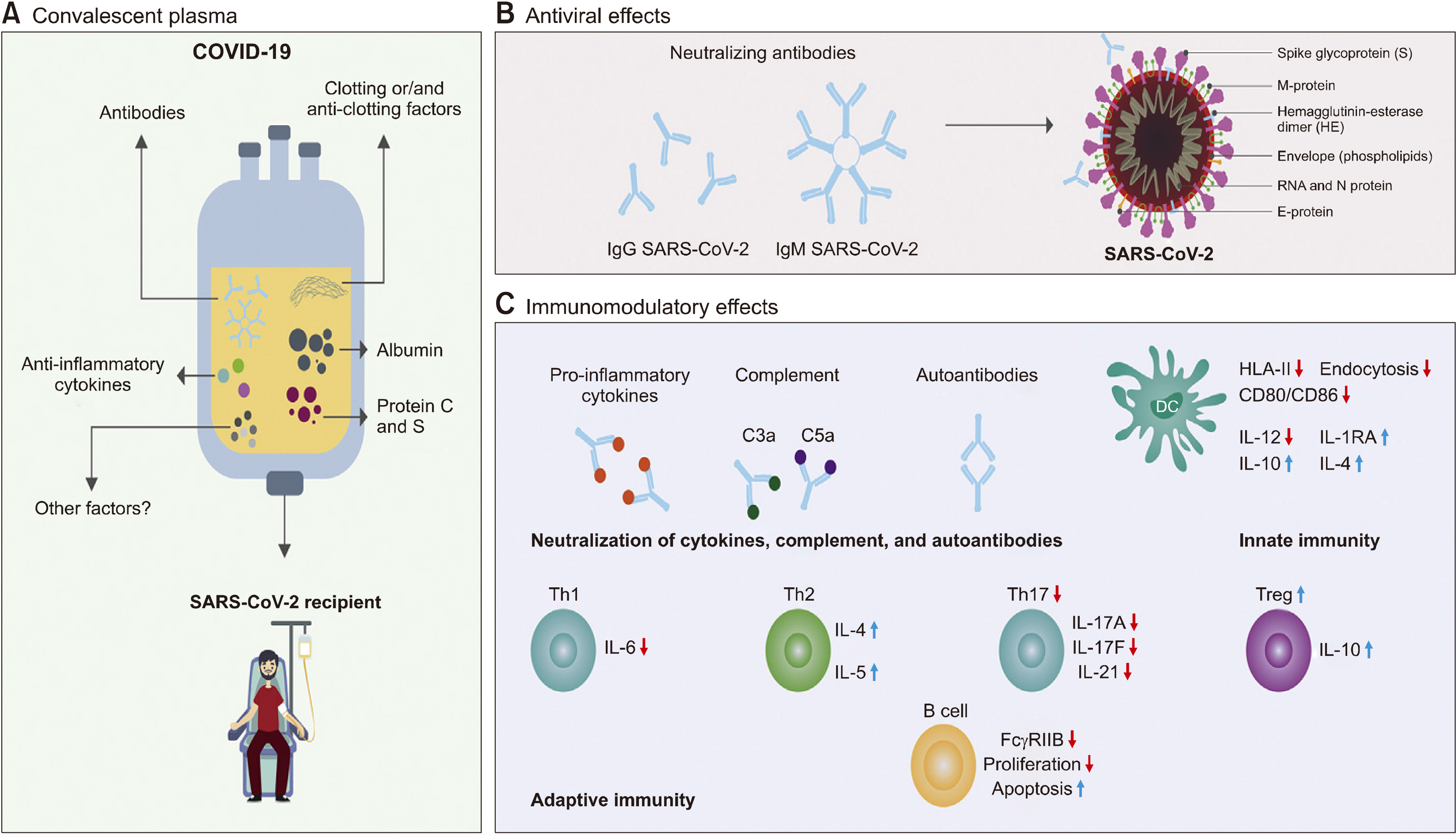
- Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for the coronavirus disease 2019 (COVID-19) pandemic that has strained health care systems worldwide and resulted in high mortality. The current COVID-19 treatment is based on supportive and symptomatic care. Therefore, convalescent plasma (CP), which provides passive immunization against many infectious diseases, has been studied for COVID-19 management. To date, a large number of randomized and non-randomized clinical trials as well as many systematic reviews have revealed conflicting results. This article summarizes the basic principles of passive immunization, particularly addressing CP in COVID-19. It also evaluates the effectiveness of CP as a therapy in patients with COVID-19, clinical trial reports and systematic reviews, regulatory considerations and different protocols that are authorized in different countries to use it safely and effectively. An advanced search was carried out in major databases (PubMed, Cochrane Library, and MEDLINE) and Google Scholar using the following key words: SARS-CoV-2, COVID-19, convalescent plasma, and the applied query was “convalescent plasma” AND “COVID-19 OR SARS-CoV-2”. The results were filtered and duplicate data were removed. Collective evidence indicates that two cardinal players determine the effectiveness of CP use, time of infusion, and quality of CP. Early administration of CP with high neutralizing anti-spike IgG titer is hypothesized to be effective in improving clinical outcome, prevent progression, decrease the length of hospital stay, and reduce mortality. However, more reliable, high quality, well-controlled, double-blinded, randomized, international and multicenter collaborative trials are still needed.
Figure
Reference
-
1. WHO Blood Regulators Network. 2017. Position paper on use of convalescent plasma, serum or immune globulin concentrates as an element in response to an emerging virus. WHO Blood Regulators Network;Geneva, Switzerland: at https://www.who.int/bloodproducts/brn/2017_BRN_PositionPaper_ConvalescentPlasma.pdf?ua=1. Accessed August 3, 2021.2. Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. 2020; Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 55:105924. DOI: 10.1016/j.ijantimicag.2020.105924. PMID: 32081636. PMCID: PMC7127800.
Article3. Wang M, Cao R, Zhang L, et al. 2020; Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 30:269–71. DOI: 10.1038/s41422-020-0282-0. PMID: 32020029. PMCID: PMC7054408.
Article4. Marano G, Vaglio S, Pupella S, et al. 2016; Convalescent plasma: new evidence for an old therapeutic tool? Blood Transfus. 14:152–7. DOI: 10.2450/2015.0131-15. PMID: 26674811. PMCID: PMC4781783.5. Rajendran K, Krishnasamy N, Rangarajan J, Rathinam J, Natarajan M, Ramachandran A. 2020; Convalescent plasma transfusion for the treatment of COVID-19: systematic review. J Med Virol. 92:1475–83. DOI: 10.1002/jmv.25961. PMID: 32356910. PMCID: PMC7267113.
Article6. Du L, He Y, Zhou Y, Liu S, Zheng BJ, Jiang S. 2009; The spike protein of SARS-CoV--a target for vaccine and therapeutic development. Nat Rev Microbiol. 7:226–36. DOI: 10.1038/nrmicro2090. PMID: 19198616. PMCID: PMC2750777.
Article7. Wu F, Wang A, Liu M, et al. 2020; Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. medRxiv. 20047365. DOI: 10.1101/2020.03.30.20047365.
Article8. Bloch EM, Shoham S, Casadevall A, et al. 2020; Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 130:2757–65. DOI: 10.1172/JCI138745. PMID: 32254064. PMCID: PMC7259988.
Article9. Hsueh PR, Huang LM, Chen PJ, Kao CL, Yang PC. 2004; Chronological evolution of IgM, IgA, IgG and neutralisation antibodies after infection with SARS-associated coronavirus. Clin Microbiol Infect. 10:1062–6. DOI: 10.1111/j.1469-0691.2004.01009.x. PMID: 15606632. PMCID: PMC7129952.
Article10. Rokni M, Ghasemi V, Tavakoli Z. 2020; Immune responses and pathogenesis of SARS-CoV-2 during an outbreak in Iran: comparison with SARS and MERS. Rev Med Virol. 30:e2107. DOI: 10.1002/rmv.2107. PMID: 32267987. PMCID: PMC7235481.
Article11. Zhang Y, Xiao M, Zhang S, et al. 2020; Coagulopathy and antiphospholipid antibodies in patients with COVID-19. N Engl J Med. 382:e38. DOI: 10.1056/NEJMc2007575. PMID: 32268022. PMCID: PMC7161262.
Article12. Abe Y, Horiuchi A, Miyake M, Kimura S. 1994; Anti-cytokine nature of natural human immunoglobulin: one possible mechanism of the clinical effect of intravenous immunoglobulin therapy. Immunol Rev. 139:5–19. DOI: 10.1111/j.1600-065X.1994.tb00854.x. PMID: 7927413.
Article13. Kulkarni R, editor. 2020. Antibody-dependent enhancement of viral infections. Dynamics of immune activation in viral diseases. Springer;Singapore: p. 9–41.
Article14. Chaigne B, Mouthon L. 2017; Mechanisms of action of intravenous immunoglobulin. Transfus Apher Sci. 56:45–9. DOI: 10.1016/j.transci.2016.12.017. PMID: 28161150.
Article15. Akilesh S, Petkova S, Sproule TJ, Shaffer DJ, Christianson GJ, Roopenian D. 2004; The MHC class I-like Fc receptor promotes humorally mediated autoimmune disease. J Clin Invest. 113:1328–33. DOI: 10.1172/JCI18838. PMID: 15124024. PMCID: PMC398424.
Article16. Jin J, Gong J, Lin B, Li Y, He Q. 2017; FcgRIIb expression on B cells is associated with treatment efficacy for acute rejection after kidney transplantation. Mol Immunol. 85:283–92. DOI: 10.1016/j.molimm.2017.03.006. PMID: 28360016.17. Aubin E, Lemieux R, Bazin R. 2010; Indirect inhibition of in vivo and in vitro T-cell responses by intravenous immunoglobulins due to impaired antigen presentation. Blood. 115:1727–34. DOI: 10.1182/blood-2009-06-225417. PMID: 19965673.
Article18. Ahmadi M, Abdolmohammadi-Vahid S, Ghaebi M, et al. 2017; Effect of intravenous immunoglobulin on Th1 and Th2 lymphocytes and improvement of pregnancy outcome in recurrent pregnancy loss (RPL). Biomed Pharmacother. 92:1095–102. DOI: 10.1016/j.biopha.2017.06.001. PMID: 28622710.
Article19. McGonagle D, Sharif K, O'Regan A, Bridgewood C. 2020; The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 19:102537. DOI: 10.1016/j.autrev.2020.102537. PMID: 32251717. PMCID: PMC7195002.
Article20. Kozicky LK, Zhao ZY, Menzies SC, et al. 2015; Intravenous immuno-globulin skews macrophages to an anti-inflammatory, IL-10-producing activation state. J Leukoc Biol. 98:983–94. DOI: 10.1189/jlb.3VMA0315-078R. PMID: 26216934.
Article21. Katz LM. 2021; (A Little) Clarity on convalescent plasma for COVID-19. N Engl J Med. 384:666–8. DOI: 10.1056/NEJMe2035678. PMID: 33440086. PMCID: PMC7821982.
Article22. 2020. Infectious Diseases Society of America. Clarifying the emergency use authorization framework for COVID-19 convalescent plasma: considerations for clinicians prepared jointly by the Infectious Diseases Society of America and AABB. Infectious Diseases Society of America;Arlington, VA: at https://www.idsociety.org/globalassets/covid-19-real-time-learning-network/therapeutics-and-interventions/convalescent-plasma/aabb-idsa-convalescent-plasma-eua--final.pdf. Accessed August 3, 2021.23. Estcourt LJ, Roberts DJ. 2020; Convalescent plasma for COVID-19. BMJ. 370:m3516. DOI: 10.1136/bmj.m3516. PMID: 32933945. PMCID: PMC7844928.
Article24. National Health Commission of the People's Republic of China. 2020. Protocol on prevention and control of COVID-19. 6th ed. National Health Commission of the People's Republic of China;Beijing, China:25. National Health Commission of the People's Republic of China. 2020. Diagnosis and treatment protocol for COVID-19. Trial Version 7. National Health Commission of the People's Republic of China;Beijing, China:26. National Health Commission of the People's Republic of China. 2020. Full text: diagnosis and treatment protocol for COVID-19 patients. Tentative 8th ed. National Health Commission of the People's Republic of China;Beijing, China:27. Chen L, Xiong J, Bao L, Shi Y. 2020; Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 20:398–400. DOI: 10.1016/S1473-3099(20)30141-9. PMID: 32113510. PMCID: PMC7128218.
Article28. University of Oxford. 2020. Randomised evaluation of COVID-19 therapy (recovery). University of Oxford;Oxford, UK: at https://clinicaltrials.gov/ct2/show/record/NCT04381936. Accessed August 3, 2021.29. Agarwal A, Mukherjee A, Kumar G, et al. 2020; Convalescent plasma in the management of moderate COVID-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ. 371:m3939. DOI: 10.1136/bmj.m3939. PMID: 33093056. PMCID: PMC7578662.
Article30. Avendaño-Solà C, Ramos-Martínez A, Muñez-Rubio E, et al. 2020; Convalescent plasma for COVID-19: a multicenter, randomized clinical trial. medRxiv. 20182444.
Article31. Gharbharan A, Jordans CCE, Geurtsvankessel C, et al. 2020; Convalescent plasma for COVID-19. A randomized clinical trial. medRxiv. 20139857.
Article32. Li L, Zhang W, Hu Y, et al. 2020; Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 324:460–70. DOI: 10.1001/jama.2020.10044. PMID: 32492084. PMCID: PMC7270883.33. Rasheed AM, Fatak DF, Hashim HA, et al. 2020; The therapeutic potential of convalescent plasma therapy on treating critically-ill COVID-19 patients residing in respiratory care units in hospitals in Baghdad, Iraq. Infez Med. 28:357–66. DOI: 10.1101/2020.06.24.20121905. PMID: 32920571.34. Libster R, Pérez Marc G, Wappner D, et al. 2021; Early high-titer plasma therapy to prevent severe COVID-19 in older adults. N Engl J Med. 384:610–8. DOI: 10.1056/NEJMoa2033700. PMID: 33406353. PMCID: PMC7793608.
Article35. Abolghasemi H, Eshghi P, Cheraghali AM, et al. 2020; Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: results of a multicenter clinical study. Transfus Apher Sci. 59:102875. DOI: 10.1016/j.transci.2020.102875. PMID: 32694043. PMCID: PMC7362821.
Article36. Salazar E, Christensen PA, Graviss EA, et al. 2020; Treatment of coronavirus disease 2019 patients with convalescent plasma reveals a signal of significantly decreased mortality. Am J Pathol. 190:2290–303. DOI: 10.1016/j.ajpath.2020.08.001. PMID: 32795424. PMCID: PMC7417901.
Article37. Joyner MJ, Wright RS, Fairweather D, et al. 2020; Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest. 130:4791–7. DOI: 10.1172/JCI140200. PMID: 32525844. PMCID: PMC7456238.38. Piechotta V, Chai KL, Valk SJ, et al. 2020; Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review. Cochrane Database Syst Rev. 7:CD013600. DOI: 10.1002/14651858.CD013600.pub2. PMID: 32648959. PMCID: PMC7389743.
Article39. Chai KL, Valk SJ, Piechotta V, et al. 2020; Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review. Cochrane Database Syst Rev. 10:CD013600. DOI: 10.1002/14651858.CD013600.pub3. PMID: 33044747.
Article40. Wang Y, Huo P, Dai R, et al. 2021; Convalescent plasma may be a possible treatment for COVID-19: a systematic review. Int Immuno-pharmacol. 91:107262. DOI: 10.1016/j.intimp.2020.107262. PMID: 33338863. PMCID: PMC7833727.
Article41. Selvi V. 2020; Convalescent plasma: a challenging tool to treat COVID-19 patients-a lesson from the past and new perspectives. Biomed Res Int. 2020:2606058. DOI: 10.1155/2020/2606058. PMID: 33029499. PMCID: PMC7512050.
Article42. Tiberghien P, de Lamballerie X, Morel P, Gallian P, Lacombe K, Yazdanpanah Y. 2020; Collecting and evaluating convalescent plasma for COVID-19 treatment: why and how? Vox Sang. 115:488–94. DOI: 10.1111/vox.12926. PMID: 32240545.
Article43. Zhao J, Yuan Q, Wang H, et al. 2020; Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin Infect Dis. 71:2027–34. DOI: 10.1093/cid/ciaa344. PMID: 32221519. PMCID: PMC7184337.
Article44. Bello-López JM, Delgado-Balbuena L, Rojas-Huidobro D, Rojo-Medina J. 2018; Treatment of platelet concentrates and plasma with riboflavin and UV light: impact in bacterial reduction. Transfus Clin Biol. 25:197–203. DOI: 10.1016/j.tracli.2018.03.004. PMID: 29656962.
Article45. Wood EM, Estcourt LJ, McQuilten ZK. 2021; How should we use convalescent plasma therapies for the management of COVID-19? Blood. 137:1573–81. DOI: 10.1182/blood.2020008903. PMID: 33202419. PMCID: PMC7992504.
Article46. Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. 2020; Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 181:281–92.e6. DOI: 10.1016/j.cell.2020.02.058. PMID: 32155444. PMCID: PMC7102599.
Article47. Wölfel R, Corman VM, Guggemos W, et al. 2020; Virological assessment of hospitalized cases of coronavirus disease 2019. medRxiv. 20030502. DOI: 10.1101/2020.03.05.20030502.
Article48. Psaltopoulou T, Sergentanis TN, Pappa V, et al. 2020; The emerging role of convalescent plasma in the treatment of COVID-19. Hemasphere. 4:e409. DOI: 10.1097/HS9.0000000000000409. PMID: 32647807. PMCID: PMC7306310. PMID: be424b77448f4f1588c149245b01a45a.
Article49. Shankar-Hari M, Estcourt L, Harvala H, Roberts D, Menon DK. United Kingdom SARS-CoV-2 Convalescent Plasma Evaluation (SCoPE) Consortium. 2020; Convalescent plasma to treat critically ill patients with COVID-19: framing the need for randomised clinical trials. Crit Care. 24:449. DOI: 10.1186/s13054-020-03163-3. PMID: 32690059. PMCID: PMC7370253. PMID: 34b9b057910344a4846a11574535683e.
Article50. Sullivan HC, Roback JD. 2020; Convalescent plasma: therapeutic hope or hopeless strategy in the SARS-CoV-2 pandemic. Transfus Med Rev. 34:145–50. DOI: 10.1016/j.tmrv.2020.04.001. PMID: 32359788. PMCID: PMC7179481.
Article51. Dzik S. 2020; COVID-19 convalescent plasma: now is the time for better science. Transfus Med Rev. 34:141–4. DOI: 10.1016/j.tmrv.2020.04.002. PMID: 32359789. PMCID: PMC7177063.
Article52. Rojas M, Rodríguez Y, Monsalve DM, et al. 2020; Convalescent plasma in COVID-19: possible mechanisms of action. Autoimmun Rev. 19:102554. DOI: 10.1016/j.autrev.2020.102554. PMID: 32380316. PMCID: PMC7198427.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Convalescent Plasma Therapy for Coronavirus Disease 2019
- Practical Considerations in Convalescent Plasma Therapy for Coronavirus Disease 2019
- Convalescent plasma therapy and remdesivir duo successfully salvaged an early liver transplant recipient with severe COVID-19 pneumonia
- Use of Convalescent Plasma Therapy in Two COVID-19 Patients with Acute Respiratory Distress Syndrome in Korea
- Convalescent Plasma Therapy in Coronavirus Disease 2019: a Case Report and Suggestions to Overcome Obstacles