Obstet Gynecol Sci.
2022 Mar;65(2):188-196. 10.5468/ogs.21313.
Incidence and predictive factors for recurrent clear cell ovarian carcinoma: results from a single center in Thailand
- Affiliations
-
- 1Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Faculty of Medicine, Prince of Songkla University, Songkhla, Thailand
- KMID: 2527270
- DOI: http://doi.org/10.5468/ogs.21313
Abstract
Objective
We aimed to study the incidence and predictive factors of recurrent clear cell ovarian carcinoma (CCC) and evaluate the oncological outcomes after recurrence.
Methods
This was a retrospective study of 134 CCC cases diagnosed between 2005 and 2020. Clinicopathological data and oncological outcomes were extracted and evaluated. Patients with co-malignancy, mixed pathological type, or incomplete data were excluded. Descriptive statistics, univariate and multivariable analyses, and Kaplan-Meier survival probability estimates were completed. A proportional hazards model was used to assess the association between the prognostic factors with progression-free survival (PFS), overall survival (OS), and post-recurrence survival.
Results
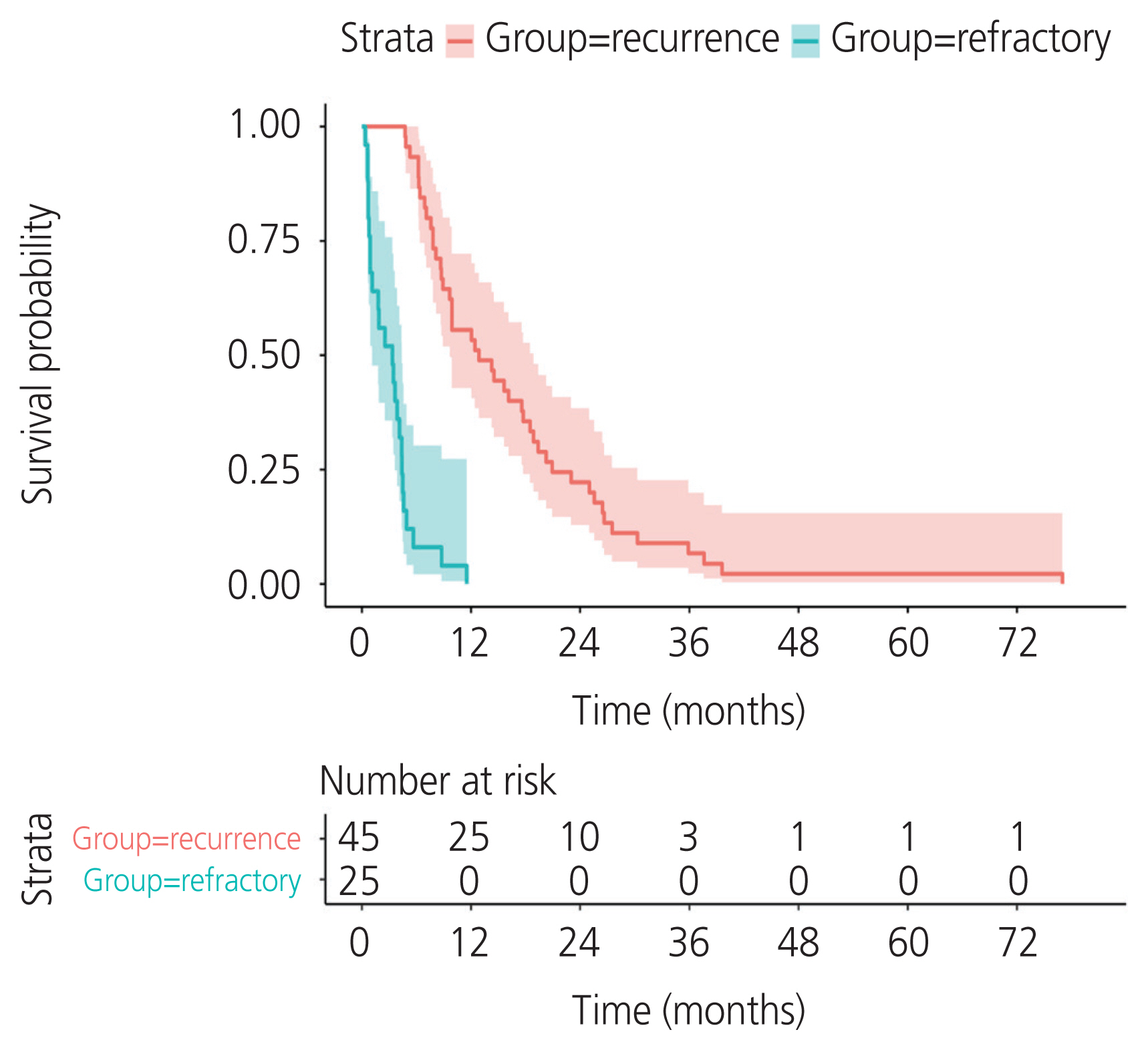
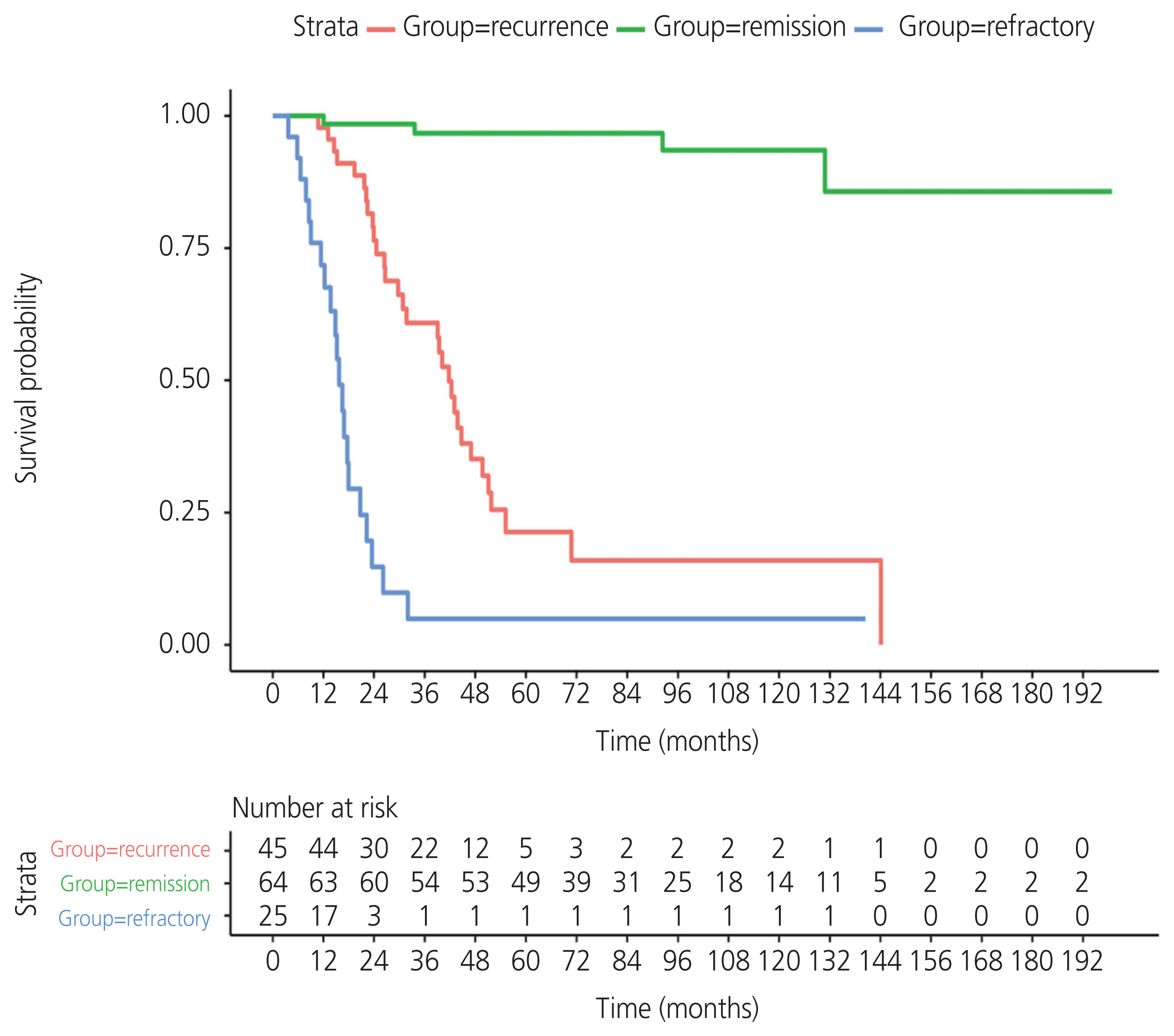
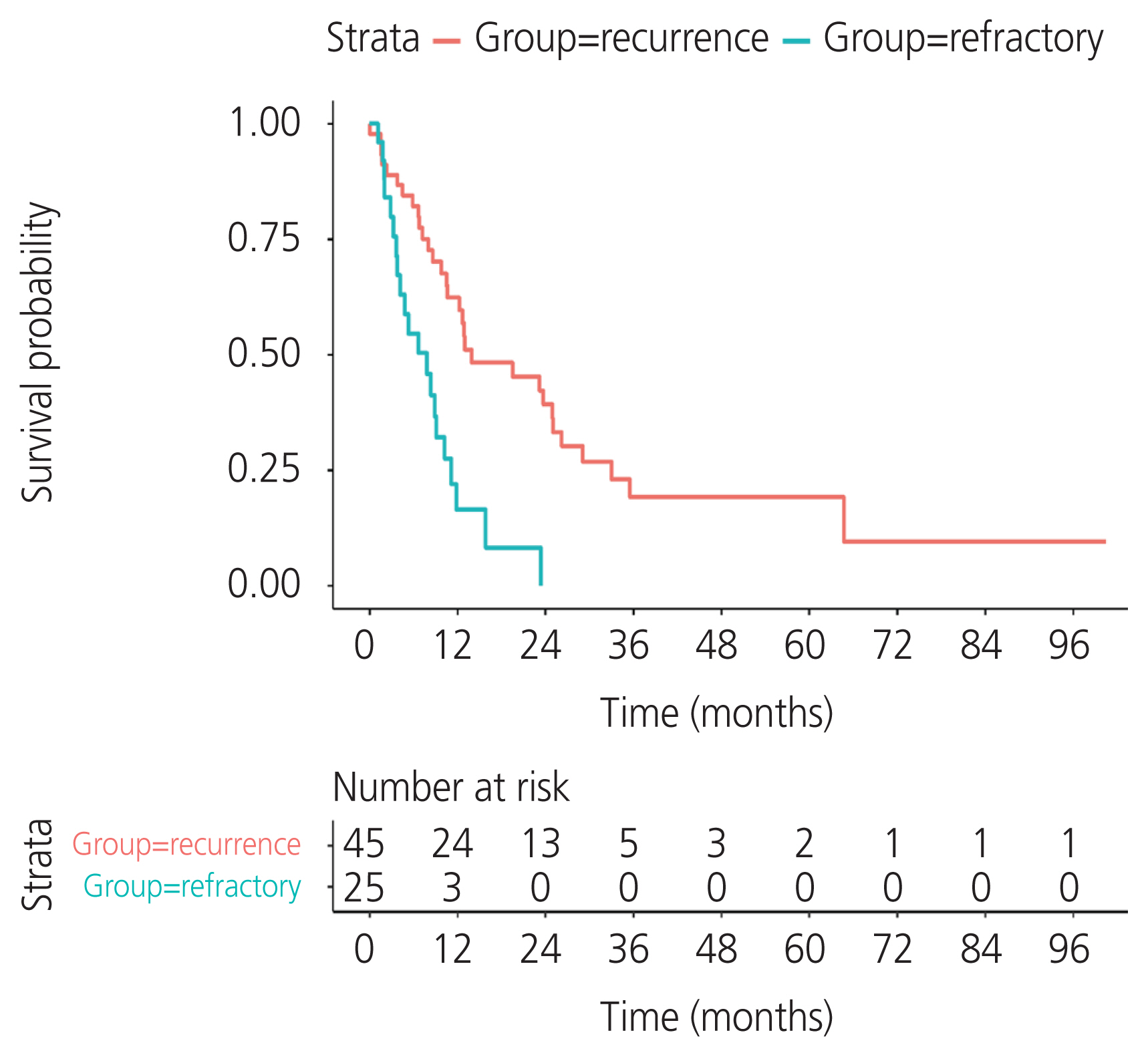
A total of 134 patients with CCC were enrolled. The incidence of recurrent CCC was 33.6% (45/134). The median PFS was 12.8 months (95% confidence interval [CI], 9.66-18.9) in the recurrence group and 3.3 months (95% CI, 1.15-4.4) in the refractory group. Residual tumor from surgical outcome, ascites cytology, and lymphovascular space invasion (LVSI) were independent prognostic factors for PFS. The significant variables were residual tumor (sub-optimal surgery vs. optimal surgery) (hazard ratio [HR], 2.68; 95% CI, 1.48-4.87; P=0.002), ascites cytology (positive vs. negative) (HR, 2.8; 95% CI, 1.58-4.98; P=0.002), and LVSI (positive vs. negative) (HR, 2.14; 95% CI, 1.18-3.86; P=0.04). The median postrecurrence survival was 13.96 months (95% CI, 10.61-26.2) in the recurrence group.
Conclusion
CCC has a high rate of recurrence. Sub-optimal surgery, positive ascites cytology, and LVSI indicated a worse prognosis for PFS. Optimal cytoreductive surgery is an important part of primary treatment to improve survival in patients with CCC.
Figure
Reference
-
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–49.
Article2. Scully RE, Young RH, Clement PB. Tumors of the ovary, maldeveloped gonads, fallopian tube, and broad ligament. Atlas of Tumor Pathology. 3rd ed. Washington DC: Armed Forces institute of Pathology;1998. p. 1–168.3. Ku FC, Wu RC, Yang LY, Tang YH, Chang WY, Yang JE, et al. Clear cell carcinomas of the ovary have poorer outcomes compared with serous carcinomas: results from a single-center Taiwanese study. J Formos Med Assoc. 2018; 117:117–25.
Article4. Ha HI, Chang HK, Park SJ, Lim J, Won YJ, Lim MC. The incidence and survival of cervical, ovarian, and endometrial cancer in Korea, 1999–2017: Korea Central Cancer Registry. Obstet Gynecol Sci. 2021; 64:444–53.
Article5. Iida Y, Okamoto A, Hollis RL, Gourley C, Herrington CS. Clear cell carcinoma of the ovary: a clinical and molecular perspective. Int J Gynecol Cancer. 2021; 31:605–16.
Article6. Lee YY, Kim TJ, Kim MJ, Kim HJ, Song T, Kim MK, et al. Prognosis of ovarian clear cell carcinoma compared to other histological subtypes: a meta-analysis. Gynecol Oncol. 2011; 122:541–7.
Article7. Colombo N, Sessa C, Du Bois A, Ledermann J, McCluggage WG, McNeish I, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann Oncol. 2019; 30:672–705.
Article8. Armstrong DK, Alvarez RD, Bakkum-Gamez JN, Barroilhet L, Behbakht K, Berchuck A, et al. Ovarian cancer, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021; 19:191–226.
Article9. Hogen L, Vicus D, Ferguson SE, Gien LT, Nofech-Mozes S, Lennox GK, et al. Patterns of recurrence and impact on survival in patients with clear cell ovarian carcinoma. Int J Gynecol Cancer. 2019; 29:1164–9.
Article10. Kajiyama H, Mizuno M, Shibata K, Umezu T, Suzuki S, Yamamoto E, et al. A recurrence-predicting prognostic factor for patients with ovarian clear-cell adenocarcinoma at reproductive age. Int J Clin Oncol. 2014; 19:921–7.
Article11. Kajiyama H, Suzuki S, Yoshihara M, Nishino K, Yoshikawa N, Utsumi F, et al. The possible existence of occult metastasis in patients with ovarian clear-cell carcinoma who underwent complete resection without any residual tumours. Oncotarget. 2018; 9:6298–307.
Article12. Hreshchyshyn MM, Park RC, Blessing JA, Norris HJ, Levy D, Lagasse LD, et al. The role of adjuvant therapy in stage I ovarian cancer. Am J Obstet Gynecol. 1980; 15:139–45.
Article13. Sugiyama T, Kamura T, Kigawa J, Terakawa N, Kikuchi Y, Kita T, et al. Clinical characteristics of clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer. 2000; 88:2584–9.
Article14. Tang H, Liu Y, Wang X, Guan L, Chen W, Jiang H, et al. Clear cell carcinoma of the ovary: clinicopathologic features and outcomes in a Chinese cohort. Medicine (Baltimore). 2018; 97:e10881.15. Zhu C, Zhu J, Qian L, Liu H, Shen Z, Wu D, et al. Clinical characteristics and prognosis of ovarian clear cell carcinoma: a 10-year retrospective study. BMC Cancer. 2021; 21:322.
Article16. Chan JK, Teoh D, Hu JM, Shin JY, Osann K, Kapp DS. Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers. Gynecol Oncol. 2008; 109:370–6.
Article17. Kudoh K, Kikuchi Y, Kita T, Tode T, Takano M, Hirata J, et al. Preoperative determination of several serum tumor markers in patients with primary epithelial ovarian carcinoma. Gynecol Obstet Invest. 1999; 47:52–7.
Article18. Liu H, Xu Y, Ji J, Dong R, Qiu H, Dai X. Prognosis of ovarian clear cell cancer compared with other epithelial cancer types: a population-based analysis. Oncol Lett. 2020; 19:1947–57.
Article19. Sugiyama T, Okamoto A, Enomoto T, Hamano T, Aotani E, Terao Y, et al. Randomized phase III trial of irinotecan plus cisplatin compared with paclitaxel plus carboplatin as first-line chemotherapy for ovarian clear cell carcinoma: JGOG3017/GCIG trial. J Clin Oncol. 2016; 34:2881–7.
Article20. Huang HJ, Yang LY, Tung HJ, Ku FC, Wu RC, Tang YH, et al. Management and clinical outcomes of patients with recurrent/progressive ovarian clear cell carcinoma. J Formos Med Assoc. 2020; 119:793–804.
Article21. Takano M, Kikuchi Y, Yaegashi N, Kuzuya K, Ueki M, Tsuda H, et al. Clear cell carcinoma of the ovary: a retrospective multicentre experience of 254 patients with complete surgical staging. Br J Cancer. 2006; 94:1369–74.
Article22. Matsuo K, Yoshino K, Hasegawa K, Murakami R, Ikeda Y, Adachi S, et al. Survival outcome of stage I ovarian clear cell carcinoma with lympho-vascular space invasion. Gynecol Oncol. 2015; 136:198–204.
Article23. Kondo E, Tabata T, Suzuki N, Aoki D, Yahata H, Kotera Y, et al. The post-progression survival of patients with recurrent or persistent ovarian clear cell carcinoma: results from a randomized phase III study in JGOG3017/GCIG. J Gynecol Oncol. 2020; 31:e94.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Two Cases of Clear Cell Carcinoma of Ovary
- Two cases of thromboembolism in clear cell ovarian adenocarcinoma
- Oxyphilic Clear Cell Carcinoma of the Ovary: A case report
- A case of ovarian metastasis of the recurrent uterine cervical cancer after adjuvant radiation therapy
- Differences of EDR Chemoresistance Assay and Prognosis between Recurrent Micropapillary Serous Ovarian Carcinoma and Serous Ovarian Carcinoma