J Korean Med Sci.
2022 Mar;37(10):e84. 10.3346/jkms.2022.37.e84.
Enhanced Hypoxia-Associated Genes in Impaired Contractility From Bladder Outlet Obstruction
- Affiliations
-
- 1Department of Urology, Soonchunhyang University Seoul Hospital, Soonchunhyang University School of Medicine, Seoul, Korea
- 2Department of Urology, Soonchunhyang University Cheonan Hospital, Soonchunhyang University School of Medicine, Cheonan, Korea
- 3College of Medicine and Medical Research Institute, Chungbuk National University, Cheongju, Korea
- KMID: 2526977
- DOI: http://doi.org/10.3346/jkms.2022.37.e84
Abstract
- Background
Hypoxia damages the bladder wall and contributes to the initiation of bladder dysfunction. The change of hypoxia is not well known in impaired bladder contractility caused by long-term bladder outlet obstruction (BOO). We aimed to find out whether hypoxia of bladder tissue is present and what signaling mechanisms are involved in the decompensated bladder in BOO.
Methods
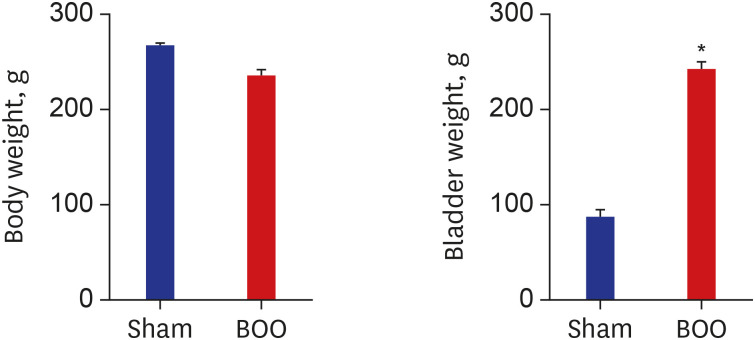
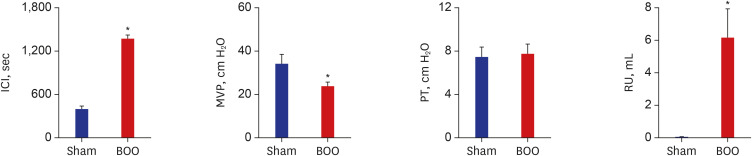
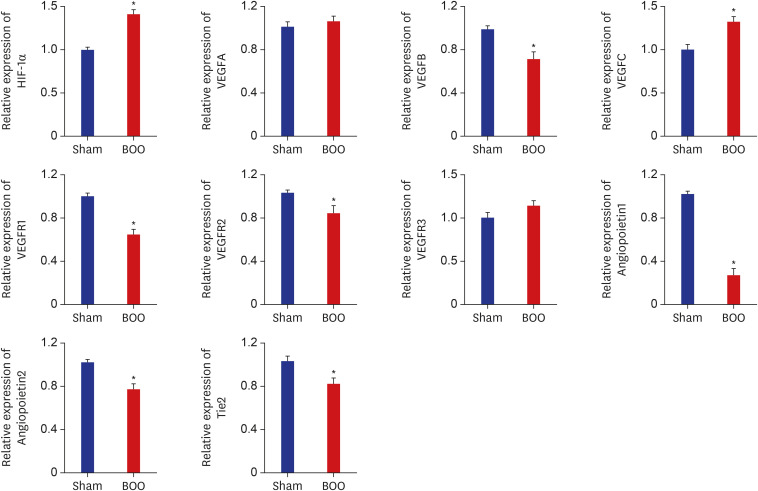
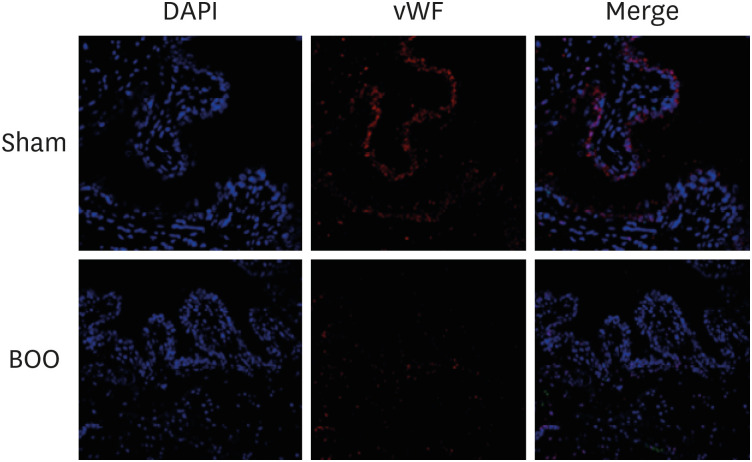
Twenty 6-week-old female Sprague-Dawley rats were divided into 2 groups, 10 rats each: group 1, sham operation; group 2, BOO for 8 weeks. Eight weeks after the onset of BOO, we did cystometric evaluation and processed polymerase chain reaction (PCR) array for hypoxia pathway using bladder tissues. The PCR array consists of 84 genes known to be involved in the hypoxic response, cell differentiation, and metabolism. We did quantitative PCR (qPCR) and immunohistochemical staining of bladder tissue for hypoxia.
Results
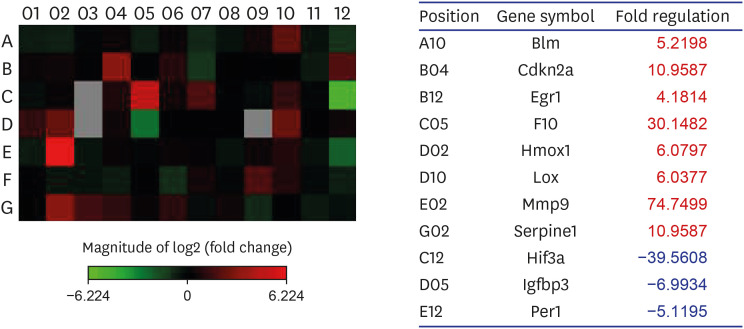
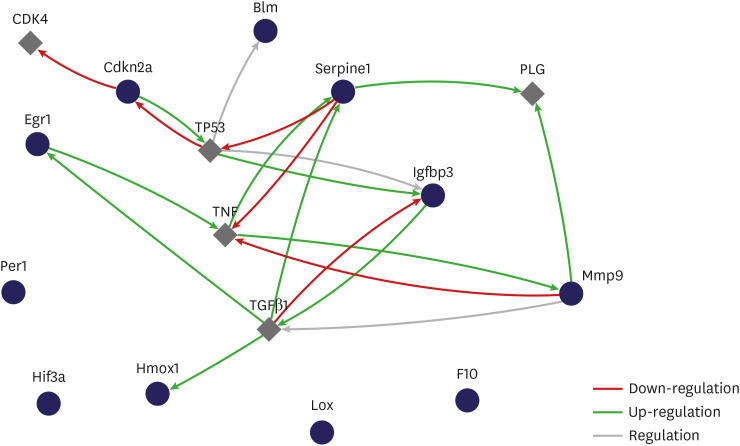
Eight genes were at least 2-fold upregulated and 3 genes were at least 2-fold downregulated in BOO group, compared with the sham operation group. The up-regulated genes (fold change) belonging to the hypoxia-inducible factor (HIF) 1 interactor included Cdkn2a (11.0), and the down-regulated genes belonging to HIF and co-transcription factors included Hif3a (−39.6) and Per1 (−5.1) by BOO. Genes influenced each other by means of TGFβ1, TNF, and TP53.
Conclusion
Hypoxia genes were increased in impaired contractility because of long-term BOO. The gene expression profiles could explain the molecular mechanisms of hypoxia in impaired contractility because of long-term BOO.
Keyword
Figure
Reference
-
1. Mehrotra RM. An experimental study of the vesical circulation during distension and in cystitis. J Pathol Bacteriol. 1953; 66(1):79–89. PMID: 13109620.
Article2. Hossler FE, Kao RL. Microvasculature of the urinary bladder of the dog: a study using vascular corrosion casting. Microsc Microanal. 2007; 13(3):220–227. PMID: 17490505.
Article3. Miodoński AJ, Litwin JA. Microvascular architecture of the human urinary bladder wall: a corrosion casting study. Anat Rec. 1999; 254(3):375–381. PMID: 10096669.
Article4. Greenland JE, Brading AF. The effect of bladder outflow obstruction on detrusor blood flow changes during the voiding cycle in conscious pigs. J Urol. 2001; 165(1):245–248. PMID: 11125418.
Article5. Azadzoi KM, Pontari M, Vlachiotis J, Siroky MB. Canine bladder blood flow and oxygenation: changes induced by filling, contraction and outlet obstruction. J Urol. 1996; 155(4):1459–1465. PMID: 8632611.
Article6. Koritsiadis G, Stravodimos K, Koutalellis G, Agrogiannis G, Koritsiadis S, Lazaris A, et al. Immunohistochemical estimation of hypoxia in human obstructed bladder and correlation with clinical variables. BJU Int. 2008; 102(3):328–332. PMID: 18384635.
Article7. Saito M, Longhurst PA, Tammela TL, Wein AJ, Levin RM. Effects of partial outlet obstruction of the rat urinary bladder on micturition characteristics, DNA synthesis and the contractile response to field stimulation and pharmacological agents. J Urol. 1993; 150(3):1045–1051. PMID: 8102184.
Article8. Brent L, Stephens FD. The response of smooth muscle cells in the rabbit urinary bladder to outflow obstruction. Invest Urol. 1975; 12(6):494–502. PMID: 1120643.9. Mostwin JL, Karim OM, van Koeveringe G, Brooks EL. The guinea pig as a model of gradual urethral obstruction. J Urol. 1991; 145(4):854–858. PMID: 2005718.
Article10. Brading A, Pessina F, Esposito L, Symes S. Effects of metabolic stress and ischaemia on the bladder, and the relationship with bladder overactivity. Scand J Urol Nephrol Suppl. 2004; 38(215):84–92.
Article11. Kato K, Monson FC, Longhurst PA, Wein AJ, Haugaard N, Levin RM. The functional effects of long-term outlet obstruction on the rabbit urinary bladder. J Urol. 1990; 143(3):600–606. PMID: 1968106.
Article12. Levin RM, Longhurst PA, Barasha B, McGuire EJ, Elbadawi A, Wein AJ. Studies on experimental bladder outlet obstruction in the cat: long-term functional effects. J Urol. 1992; 148(3):939–943. PMID: 1512863.
Article13. Greenland JE, Hvistendahl JJ, Andersen H, Jörgensen TM, McMurray G, Cortina-Borja M, et al. The effect of bladder outlet obstruction on tissue oxygen tension and blood flow in the pig bladder. BJU Int. 2000; 85(9):1109–1114. PMID: 10848706.
Article14. Levin RM, Yu HJ, Kim KB, Longhurst PA, Wein AJ, Damaser MS. Etiology of bladder dysfunction secondary to partial outlet obstruction. Calcium disregulation in bladder power generation and the ability to perform work. Scand J Urol Nephrol Suppl. 1997; 184:43–50. PMID: 9165622.15. Schröder A, Chichester P, Kogan BA, Longhurst PA, Lieb J, Das AK, et al. Effect of chronic bladder outlet obstruction on blood flow of the rabbit bladder. J Urol. 2001; 165(2):640–646. PMID: 11176451.
Article16. Gosling JA, Kung LS, Dixon JS, Horan P, Whitbeck C, Levin RM. Correlation between the structure and function of the rabbit urinary bladder following partial outlet obstruction. J Urol. 2000; 163(4):1349–1356. PMID: 10737542.
Article17. Hoskin PJ, Sibtain A, Daley FM, Wilson GD. GLUT1 and CAIX as intrinsic markers of hypoxia in bladder cancer: relationship with vascularity and proliferation as predictors of outcome of ARCON. Br J Cancer. 2003; 89(7):1290–1297. PMID: 14520462.
Article18. Lee JW, Bae SH, Jeong JW, Kim SH, Kim KW. Hypoxia-inducible factor (HIF-1)α: its protein stability and biological functions. Exp Mol Med. 2004; 36(1):1–12. PMID: 15031665.
Article19. Hoppeler H, Vogt M. Muscle tissue adaptations to hypoxia. J Exp Biol. 2001; 204(Pt 18):3133–3139. PMID: 11581327.
Article20. Semenza GL. HIF-1 and mechanisms of hypoxia sensing. Curr Opin Cell Biol. 2001; 13(2):167–171. PMID: 11248550.
Article21. Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci U S A. 1998; 95(14):7987–7992. PMID: 9653127.
Article22. Liu P, Hock CE, Nagele R, Wong PY. Formation of nitric oxide, superoxide, and peroxynitrite in myocardial ischemia-reperfusion injury in rats. Am J Physiol. 1997; 272(5 Pt 2):H2327–H2336. PMID: 9176302.
Article23. Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007; 117(12):3810–3820. PMID: 18037992.
Article24. Aitken KJ, Bägli DJ. The bladder extracellular matrix. Part I: architecture, development and disease. Nat Rev Urol. 2009; 6(11):596–611. PMID: 19890339.
Article25. Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007; 8(3):221–233. PMID: 17318226.
Article26. Karow JK, Chakraverty RK, Hickson ID. The Bloom’s syndrome gene product is a 3′-5′ DNA helicase. J Biol Chem. 1997; 272(49):30611–30614. PMID: 9388193.
Article27. Barabási AL, Oltvai ZN. Network biology: understanding the cell’s functional organization. Nat Rev Genet. 2004; 5(2):101–113. PMID: 14735121.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Clinical Significance of Detrusor Contraction Duration as a Predicting Parameter for Evaluaing Bladder Outlet Obstruction with Lower Urinary Symptoms in Men
- Anti-fibrotic effect of tocotrienols for bladder dysfunction due to partial bladder outlet obstruction
- Reversibility of Detrusor Contractility after Relief of Bladder Outlet Obstruction in the Rat
- Ultrastructural Changes of Detrusor Muscle by Partial Obstruction of the Bladder Outlet in the Rat
- Bladder Outlet Obstruction in the Female Overactive Bladder: Correct Diagnostic Criteria for Bladder Outlet Obstruction?