J Korean Med Sci.
2022 Feb;37(8):e65. 10.3346/jkms.2022.37.e65.
Soluble ACE2 and TMPRSS2 Levels in the Serum of Asthmatic Patients
- Affiliations
-
- 1Department of Allergy and Clinical Immunology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Division of Pulmonary, Allergy and Critical Care Medicine, Department of Internal Medicine, Gangneung Asan Hospital, Gangneung, Korea
- 3Department of Pulmonary and Critical Care Medicine, Kyung Hee University Hospital at Gangdong, College of Medicine, Kyung Hee University, Seoul, Korea
- 4Department of Statistics and Data Science, Korea National Open University, Seoul, Korea
- KMID: 2526797
- DOI: http://doi.org/10.3346/jkms.2022.37.e65
Abstract
- Background
Angiotensin-converting enzyme 2 (ACE2) and transmembrane protease serine subtype 2 (TMPRSS2) are key proteins mediating viral entry of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Although gene expressions of ACE2 and TMPRSS2 have been analyzed in various organs and diseases, their soluble forms have been less studied, particularly in asthma. Therefore, we aimed to measure circulating ACE2 and TMPRSS2 in the serum of asthmatics and examine their relationship with clinical characteristics.
Methods
Clinical data and serum samples of 400 participants were obtained from an asthma cohort. The soluble ACE2 (sACE2) and soluble TMPRSS2 (sTMPRSS2) level was measured by enzyme-linked immunosorbent assay, and the values underwent a natural log transformation. Associations between sACE2 and TMPRSS2 levels and various clinical variables were analyzed.
Results
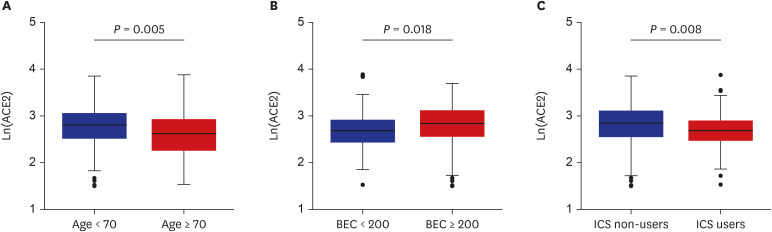
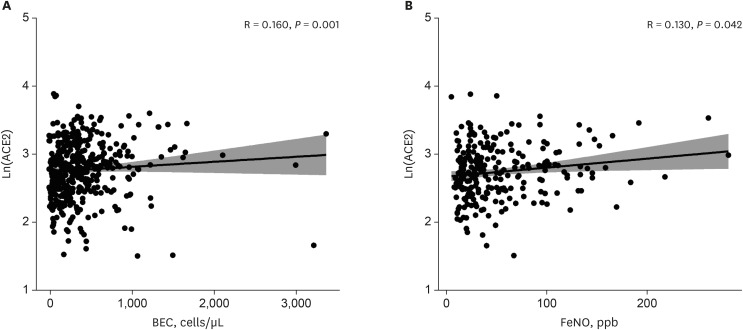
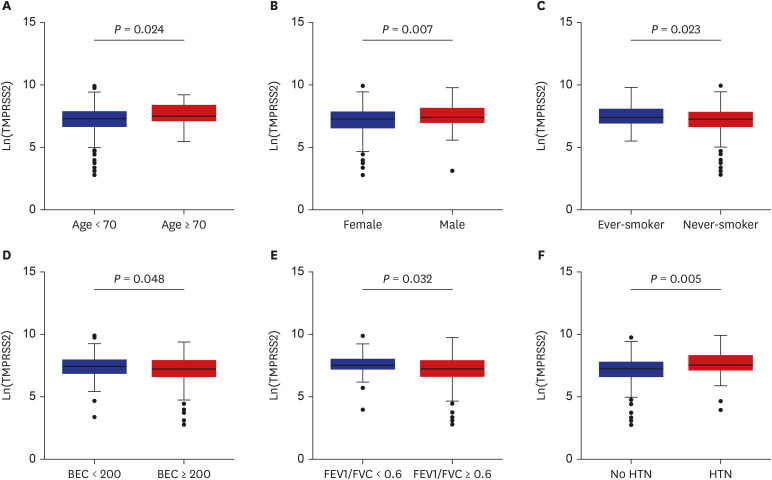
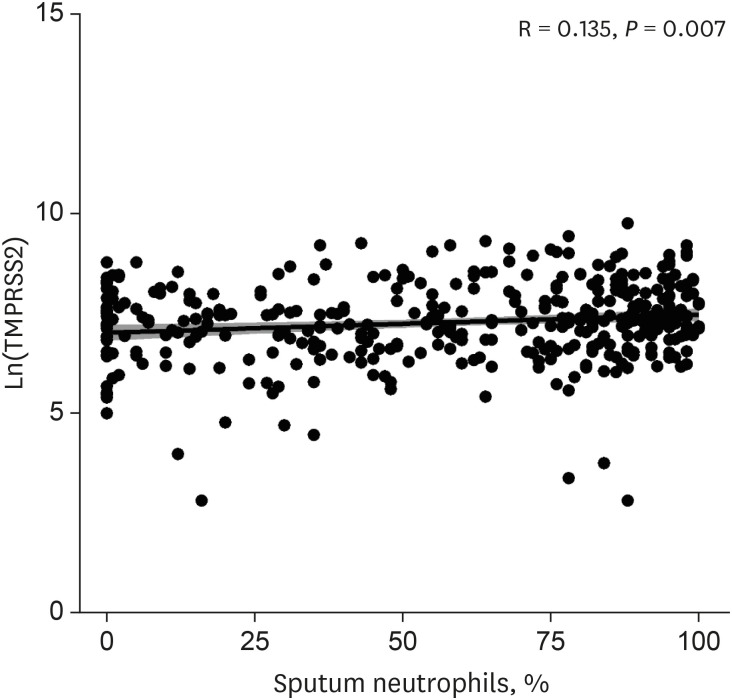
The patients younger than 70 years old, those with eosinophilic asthma (eosinophils ≥ 200 cells/µL), and inhaled corticosteroids (ICS) non-users were associated with higher levels of sACE2. Blood eosinophils and fractionated exhaled nitric oxide levels were positively correlated with serum ACE2. In contrast, lower levels of sTMPRSS2 were noted in patients below 70 years and those with eosinophilic asthma, while no association was noted between ICS use and sTMPRSS2. The level of sTMPRSS2 also differed according to sex, smoking history, coexisting hypertension, and forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) ratio. The proportion of sputum neutrophils was positively correlated with sTMPRSS2, while the FEV1/FVC ratio reported a negative correlation with sTMPRSS2.
Conclusion
The levels of ACE2 and TMPRSS2 were differently expressed according to age, ICS use, and several inflammatory markers. These findings suggest variable susceptibility and prognosis of SARS-CoV-2 infection among asthmatic patients.
Figure
Reference
-
1. Sanchez-Ramirez DC, Mackey D. Underlying respiratory diseases, specifically COPD, and smoking are associated with severe COVID-19 outcomes: a systematic review and meta-analysis. Respir Med. 2020; 171:106096. PMID: 32763754.
Article2. Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020; 180(7):934–943. PMID: 32167524.
Article3. Terry PD, Heidel RE, Dhand R. Asthma in adult patients with COVID-19. Prevalence and risk of severe disease. Am J Respir Crit Care Med. 2021; 203(7):893–905. PMID: 33493416.
Article4. Lee H, Choi S, Park JY, Jo DS, Choi UY, Lee H, et al. Analysis of critical COVID-19 cases among children in Korea. J Korean Med Sci. 2022; 37(1):e13. PMID: 34981683.
Article5. Yao Y, Wang H, Liu Z. Expression of ACE2 in airways: implication for COVID-19 risk and disease management in patients with chronic inflammatory respiratory diseases. Clin Exp Allergy. 2020; 50(12):1313–1324. PMID: 32975865.
Article6. Jackson DJ, Busse WW, Bacharier LB, Kattan M, O’Connor GT, Wood RA, et al. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor ACE2. J Allergy Clin Immunol. 2020; 146(1):203–206.e3. PMID: 32333915.
Article7. Camiolo M, Gauthier M, Kaminski N, Ray A, Wenzel SE. Expression of SARS-CoV-2 receptor ACE2 and coincident host response signature varies by asthma inflammatory phenotype. J Allergy Clin Immunol. 2020; 146(2):315–324.e7. PMID: 32531372.
Article8. Emilsson V, Gudmundsson EF, Aspelund T, Jonsson BG, Gudjonsson A, Launer LJ, et al. Serum levels of ACE2 are higher in patients with obesity and diabetes. Obes Sci Pract. 2020; 7(2):239–243. PMID: 33841894.
Article9. Swärd P, Edsfeldt A, Reepalu A, Jehpsson L, Rosengren BE, Karlsson MK. Age and sex differences in soluble ACE2 may give insights for COVID-19. Crit Care. 2020; 24(1):221. PMID: 32410690.
Article10. Reindl-Schwaighofer R, Hödlmoser S, Eskandary F, Poglitsch M, Bonderman D, Strassl R, et al. ACE2 elevation in severe COVID-19. Am J Respir Crit Care Med. 2021; 203(9):1191–1196. PMID: 33600742.
Article11. Nagy B Jr, Fejes Z, Szentkereszty Z, Sütő R, Várkonyi I, Ajzner É, et al. A dramatic rise in serum ACE2 activity in a critically ill COVID-19 patient. Int J Infect Dis. 2021; 103:412–414. PMID: 33249290.
Article12. Zoufaly A, Poglitsch M, Aberle JH, Hoepler W, Seitz T, Traugott M, et al. Human recombinant soluble ACE2 in severe COVID-19. Lancet Respir Med. 2020; 8(11):1154–1158. PMID: 33131609.
Article13. Kim TB, Park CS, Bae YJ, Cho YS, Moon HB. COREA Study Group. Factors associated with severity and exacerbation of asthma: a baseline analysis of the cohort for reality and evolution of adult asthma in Korea (COREA). Ann Allergy Asthma Immunol. 2009; 103(4):311–317. PMID: 19852195.
Article14. Kim TB, Jang AS, Kwon HS, Park JS, Chang YS, Cho SH, et al. Identification of asthma clusters in two independent Korean adult asthma cohorts. Eur Respir J. 2013; 41(6):1308–1314. PMID: 23060627.
Article15. Park SY, Jung HW, Lee JM, Shin B, Kim HJ, Kim MH, et al. Novel trajectories for identifying asthma phenotypes: a longitudinal study in Korean asthma cohort, COREA. J Allergy Clin Immunol Pract. 2019; 7(6):1850–1857.e4. PMID: 30794966.
Article16. Global Initiative for Asthma. Global strategy for asthma management and prevention. Updated 2020. Accessed October 11, 2021. https://ginasthma.org/wp-content/uploads/2020/06/GINA-2020-report_20_06_04-1-wms.pdf .17. Kornilov SA, Lucas I, Jade K, Dai CL, Lovejoy JC, Magis AT. Plasma levels of soluble ACE2are associated with sex, metabolic syndrome, and its biomarkers in a large cohort, pointing to a possible mechanism for increased severity in COVID-19. Crit Care. 2020; 24(1):452. PMID: 32698840.
Article18. Pavel AB, Wu J, Renert-Yuval Y, Del Duca E, Glickman JW, Miller RL, et al. SARS-CoV-2 receptor ACE2 protein expression in serum is significantly associated with age. Allergy. 2021; 76(3):875–878. PMID: 32726474.
Article19. AlGhatrif M, Tanaka T, Moore AZ, Bandinelli S, Lakatta EG, Ferrucci L. Age-associated difference in circulating ACE2, the gateway for SARS-COV-2, in humans: results from the InCHIANTI study. Geroscience. 2021; 43(2):619–627. PMID: 33462706.
Article20. Kermani NZ, Song WJ, Badi Y, Versi A, Guo Y, Sun K, et al. Sputum ACE2, TMPRSS2 and FURIN gene expression in severe neutrophilic asthma. Respir Res. 2021; 22(1):10. PMID: 33413387.
Article21. Peters MC, Sajuthi S, Deford P, Christenson S, Rios CL, Montgomery MT, et al. COVID-19-related genes in sputum cells in asthma. Relationship to demographic features and corticosteroids. Am J Respir Crit Care Med. 2020; 202(1):83–90. PMID: 32348692.
Article22. Kimura H, Francisco D, Conway M, Martinez FD, Vercelli D, Polverino F, et al. Type 2 inflammation modulates ACE2 and TMPRSS2 in airway epithelial cells. J Allergy Clin Immunol. 2020; 146(1):80–88.e8. PMID: 32422146.
Article23. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020; 181(2):271–280.e8. PMID: 32142651.
Article24. Davidson AM, Wysocki J, Batlle D. Interaction of SARS-CoV-2 and other coronavirus with ACE (angiotensin-converting enzyme)-2 as their main receptor: therapeutic implications. Hypertension. 2020; 76(5):1339–1349. PMID: 32851855.
Article25. Monteil V, Kwon H, Prado P, Hagelkrüys A, Wimmer RA, Stahl M, et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020; 181(4):905–913.e7. PMID: 32333836.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Genetic Susceptibility of ACE2 and TMPRSS2 in Six Common Cancers and Possible Impacts on COVID-19
- Role of Genetic Variants and Gene Expression in the Susceptibility and Severity of COVID-19
- The impact of COVID-19 on human reproduction and directions for fertility treatment during the pandemic
- Levels Serum Soluble CD25 , CD8 , and CD4 In Patients with Leprosy
- Increased serum soluble ST2 in asthmatic children and recurrent early wheezers