J Dent Rehabil Appl Sci.
2021 Dec;37(4):244-250. 10.14368/jdras.2021.37.4.244.
Characteristics of antimicrobial activity of Streptococcus salivarius K12 by culture condition
- Affiliations
-
- 1Department of Prosthodontics, College of Dentistry, Dankook University, Cheonan, Republic of Korea
- 2Department of Oral Microbiology and Immunology, College of Dentistry, Dankook University, Cheonan, Republic of Korea
- KMID: 2525999
- DOI: http://doi.org/10.14368/jdras.2021.37.4.244
Abstract
- Purpose
The purpose of this study was to examine effects of culture conditions on the growth and antibacterial activity of Streptococcus salivarius K12.
Materials and Methods
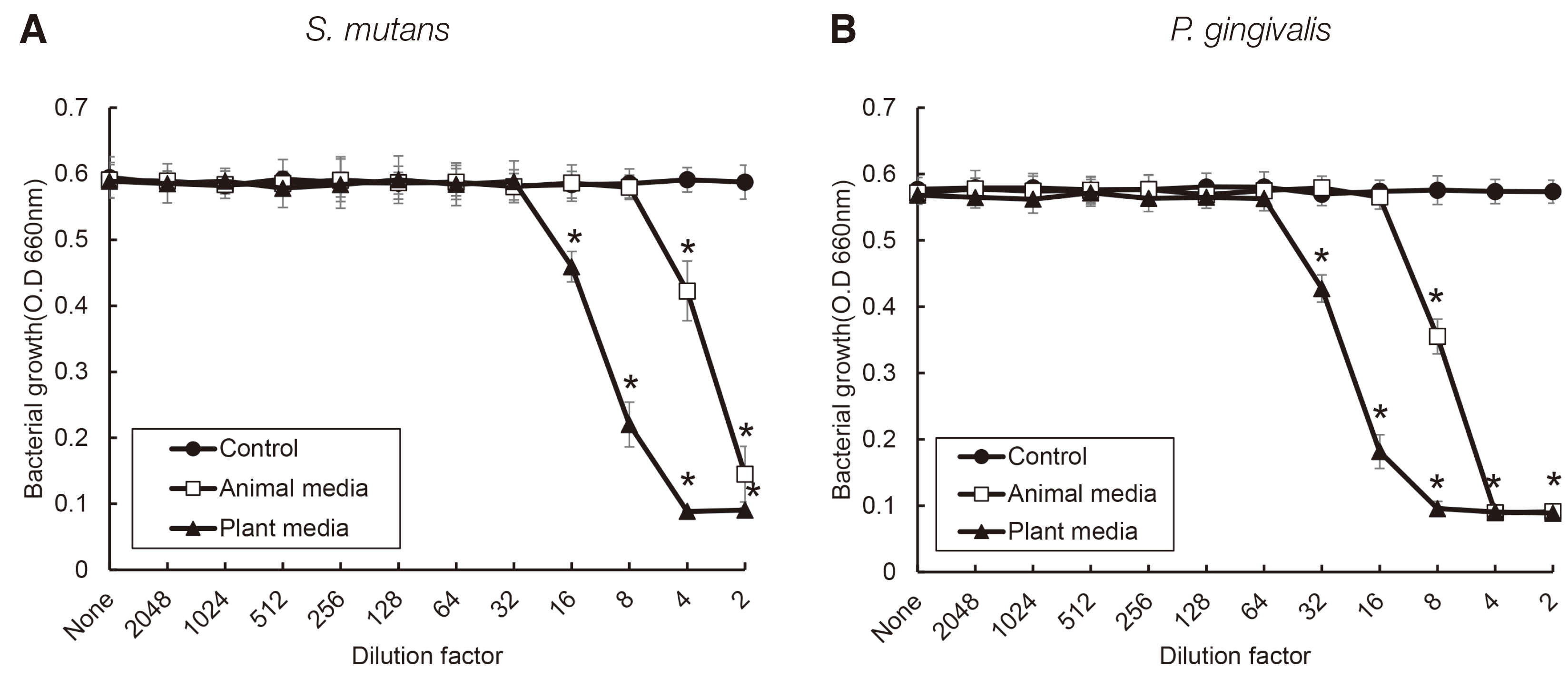
S. salivarius K12 was cultivated in medium containing animal and plant protein or in medium of neutral and acidic conditions. The growth of S. salivarius K12 was measured every 2 hours by a spectrophotometer. The antimicrobial activity of S. salivarius K12 against Streptococcus mutans and Porphyromonas gingivalis was investigated by the susceptibility assay using the spent culture medium.
Results
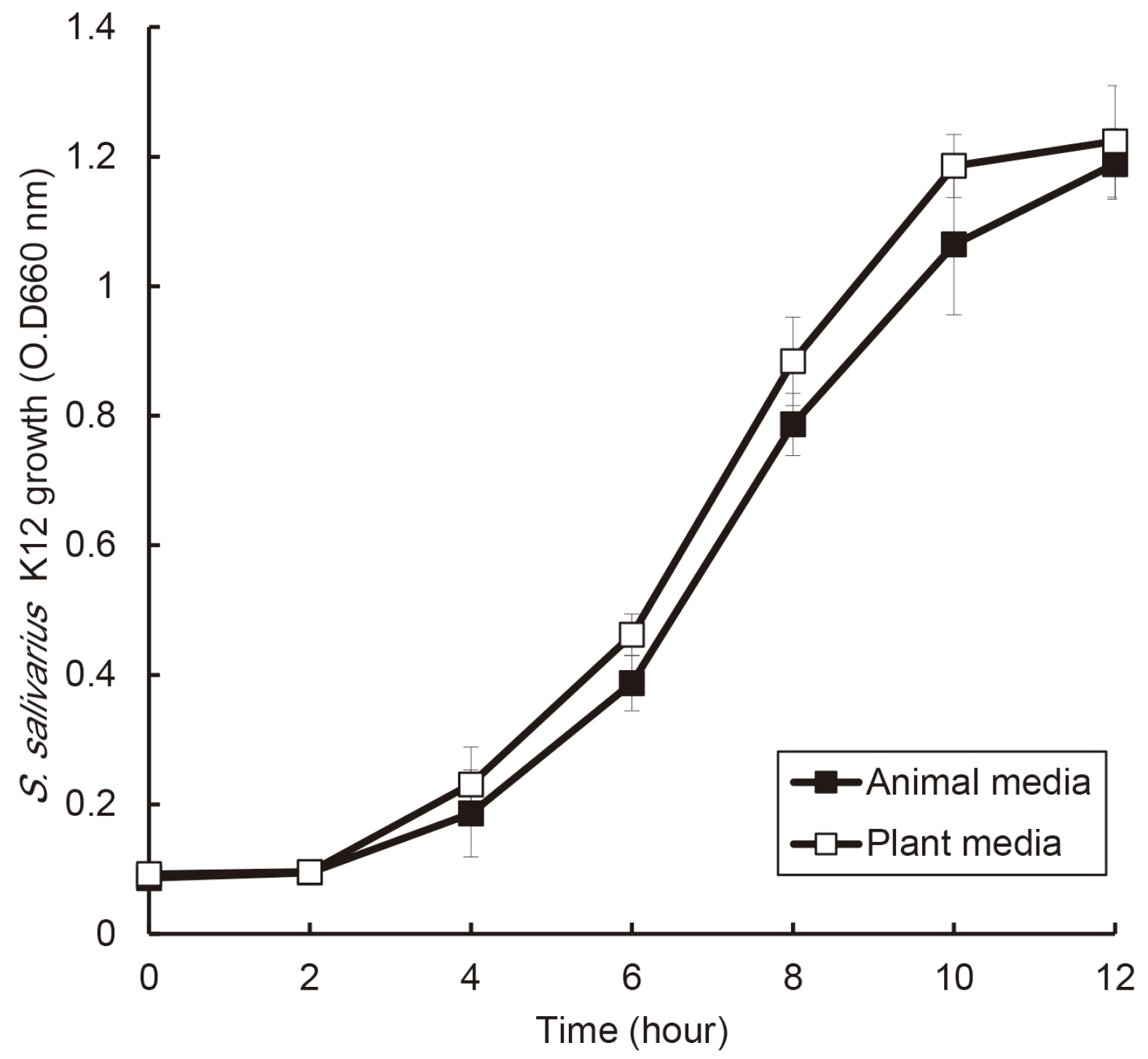
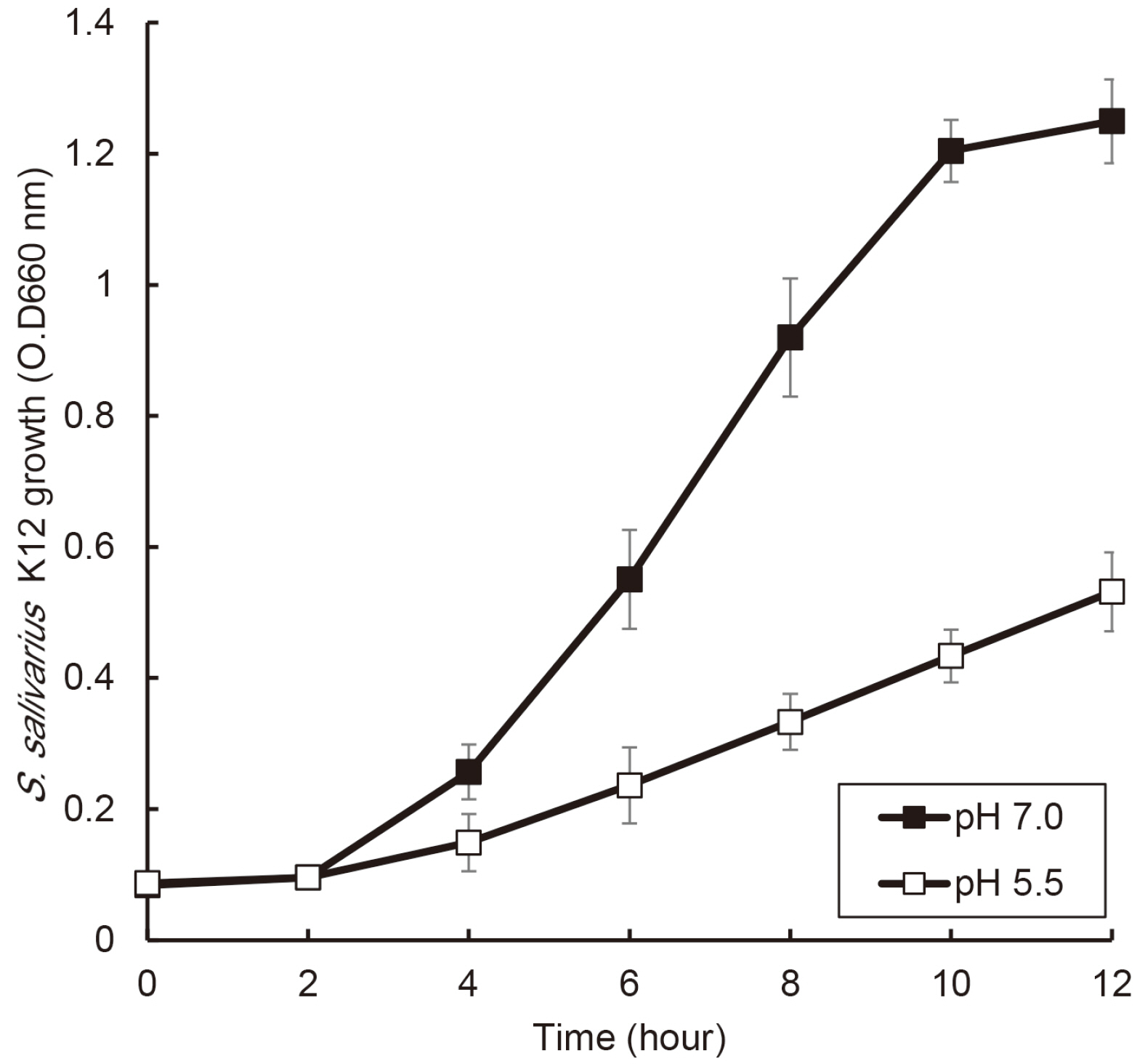
the growth of S. salivarius K12 showed faster in medium containing plant protein and neutral pH condition. The antimicrobial and antifungal activity of S. salivarius K12 appeared stronger in medium containing plant protein than animal proteins.
Conclusion
For application of S. salivarius K12 to bacterial oral disease, co-substances may be needed for S. salivarius K12 to colonize in the oral cavity and enhance the antimicrobial activity.
Figure
Reference
-
References
1. Marsh PD. 1994; Microbial ecology of dental plaque and its significance in health and disease. Adv Dent Res. 8:263–71. DOI: 10.1177/08959374940080022001. PMID: 7865085.2. Song YG, Lee SH. 2017; Inhibitory effects of Lactobacillus rhamnosus and Lactobacillus casei on Candida biofilm of denture surface. Arch Oral Biol. 76:1–6. DOI: 10.1016/j.archoralbio.2016.12.014. PMID: 28063305.3. Holt SC, Ebersole JL. 2005; Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the "red complex", a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000. 38:72–122. DOI: 10.1111/j.1600-0757.2005.00113.x. PMID: 15853938.4. Schoilew K, Ueffing H, Dalpke A, Wolff B, Frese C, Wolff D, Boutin S. 2019; Bacterial biofilm composition in healthy subjects with and without caries experience. J Oral Microbiol. 11:1633194. DOI: 10.1080/20002297.2019.1633194. PMID: 31275531. PMCID: PMC6598481.5. Velsko IM, Shaddox LM. 2018; Consistent and reproducible long-term in vitro growth of health and disease-associated oral subgingival biofilms. BMC Microbiol. 18:70. DOI: 10.1186/s12866-018-1212-x. PMID: 29996764. PMCID: PMC6042318.6. Shaddox LM, Spencer WP, Velsko IM, Al-Kassab H, Huang H, Calderon N, Aukhil I, Wallet SM. 2016; Localized aggressive periodontitis immune response to healthy and diseased subgingival plaque. J Clin Periodontol. 43:746–53. DOI: 10.1111/jcpe.12560. PMID: 27037664. PMCID: PMC4975966.7. Lee SH, Kim YJ. 2014; A comparative study of the effect of probiotics on cariogenic biofilm model for preventing dental caries. Arch Microbiol. 196:601–9. DOI: 10.1007/s00203-014-0998-7. PMID: 24919536.8. Mah TF, O'oole GA. 2001; Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 9:34–9. DOI: 10.1016/S0966-842X(00)01913-2. PMID: 11166241.9. Gill H, Prasad J. 2008; Probiotics, immunomodulation, and health benefits. Adv Exp Med Biol. 606:423–54. DOI: 10.1007/978-0-387-74087-4_17. PMID: 18183940.10. Rath H, Feng D, Neuweiler I, Stumpp NS, Nackenhorst U, Stiesch M. 2017; Biofilm formation by the oral pioneer colonizer Streptococcus gordonii: an experimental and numerical study. FEMS Microbiol Ecol. 93:doi: 10.1093/femsec/fix010. DOI: 10.1093/femsec/fix010. PMID: 28158402.11. Hyink O, Wescombe PA, Upton M, Ragland N, Burton JP, Tagg JR. 2007; Salivaricin A2 and the novel lantibiotic salivaricin B are encoded at adjacent loci on a 190-kilobase transmissible megaplasmid in the oral probiotic strain Streptococcus salivarius K12. Appl Environ Microbiol. 73:1107–13. DOI: 10.1128/AEM.02265-06. PMID: 17194838. PMCID: PMC1828679.12. Davani-Davari D, Negahdaripour M, Karimzadeh I, Seifan M, Mohkam M, Masoumi SJ, Berenjian A, Ghasemi Y. 2019; Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods. 8:92. DOI: 10.3390/foods8030092. PMID: 30857316. PMCID: PMC6463098.13. Moon YM, Moon JE, Lee MR, Cho JW. 2016; Antibacterial Effects of Streptococcus Salivarius K12 on Oral Bacteria. Int J Clin Prev Dent. 12:209–20. DOI: 10.15236/ijcpd.2016.12.4.209.14. Tagg JR. 2004; Prevention of streptococcal pharyngitis by anti-Streptococcus pyogenes bacteriocin-like inhibitory substances (BLIS) produced by Streptococcus salivarius. Indian J Med Res. 119:13–6. PMID: 15232154.15. Burton JP, Chilcott CN, Wescombe PA, Tagg JR. 2010; Extended Safety Data for the Oral Cavity Probiotic Streptococcus salivarius K12. Probiotics Antimicrob Proteins. 2:135–44. DOI: 10.1007/s12602-010-9045-4. PMID: 26781236.16. Di Pierro F, Adami T, Rapacioli G, Giardini N, Streitberger C. 2013; Clinical evaluation of the oral probiotic Streptococcus salivarius K12 in the prevention of recurrent pharyngitis and/or tonsillitis caused by Streptococcus pyogenes in adults. Expert Opin Biol Ther. 13:339–43. DOI: 10.1517/14712598.2013.758711. PMID: 23286823.17. Mokhtar M, Rismayuddin NAR, Yassim ASM, Ahmad H, Wahab RA, Dashper S, Arzmi MH. 2021; Streptococcus salivarius K12 inhibits Candida albicans aggregation, biofilm formation and dimorphism. Biofouling. 37:767–76. DOI: 10.1080/08927014.2021.1967334. PMID: 34425729.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antimicrobial activity of candidate probiotic Streptococcus salivarius against Gram-positive bacteria in oral cavity
- Antimicrobial susceptibility test on streptococcus viridans in children's oral cavity
- Antimicrobial effect of topical local anesthetic spray on oral microflora
- Streptococcus salivarius pneumonia with pulmonary nocardiosis in a rheumatoid arthritis patient treated with immunosuppressants
- Antimicrobial Effect of Acanthopanax sessiliflorum Fruit Extracts against Selected Oral Bacteria