J Korean Acad Oral Health.
2022 Dec;46(4):217-221. 10.11149/jkaoh.2022.46.4.217.
Antimicrobial activity of candidate probiotic Streptococcus salivarius against Gram-positive bacteria in oral cavity
- Affiliations
-
- 1Department of Oral Microbiology and Immunology, College of Dentistry, Dankook University, Cheonan, Korea
- KMID: 2537817
- DOI: http://doi.org/10.11149/jkaoh.2022.46.4.217
Abstract
Objectives
The aim of this study is to investigate antimicrobial activity in isolated Streptococcus salivarius against Gram-positive bacteria related oral diseases.
Methods
S. salivarius was used in G2, G7, K12, and ATCC 7073 strains and tryptic soy broth supplemented with glucose was cultivated. Actinomyces israelii, Actinomyces viscosus, and Enterococcus faecalis were cultivated with brain heart infusion broth. Streptococcus mutans and Streptococcus sobrinus were maintained using tryptic soy broth. The antimicrobial activity of S. salivarius was performed by minimum inhibitory concentration using the spent culture medium.
Results
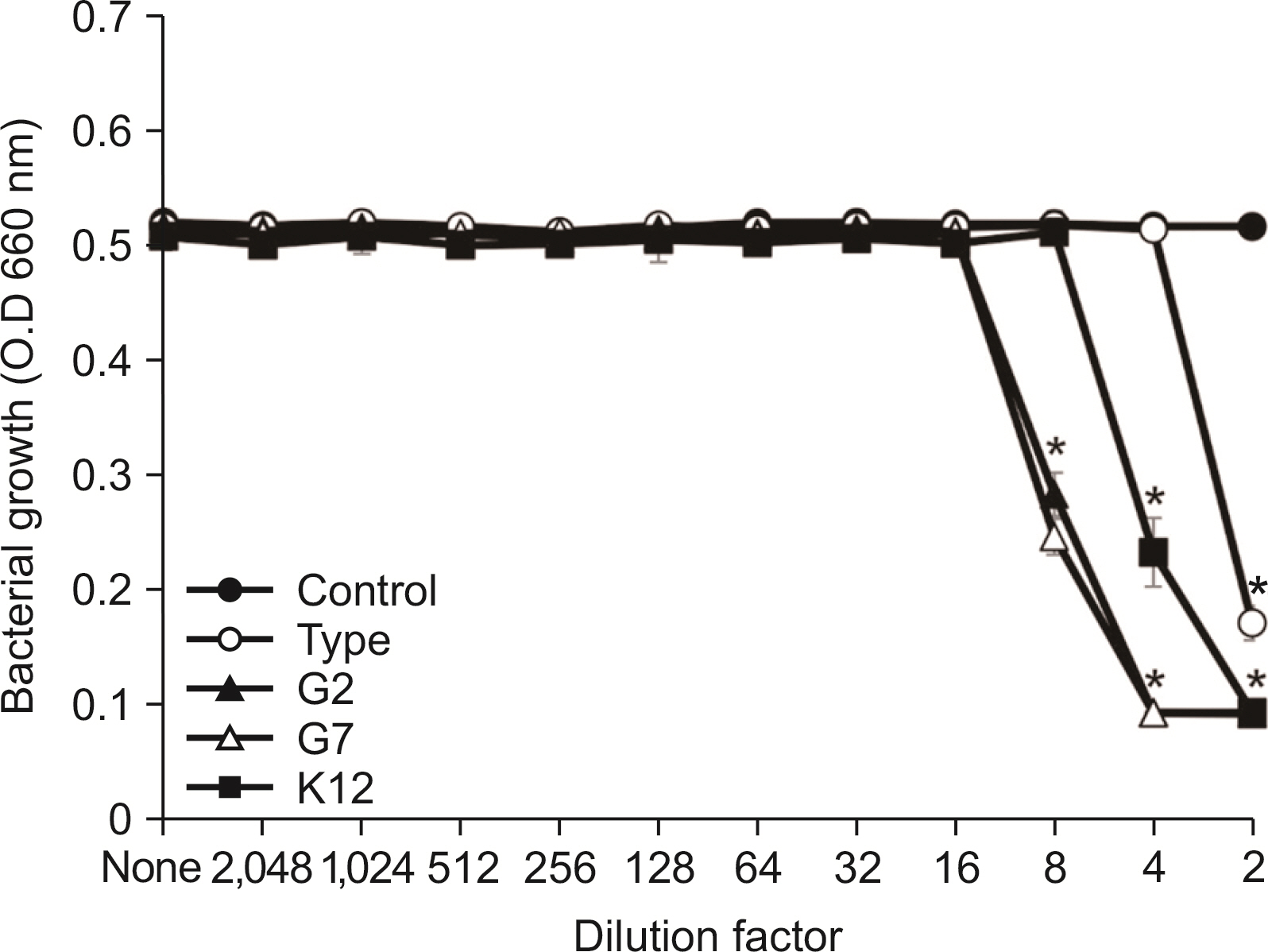
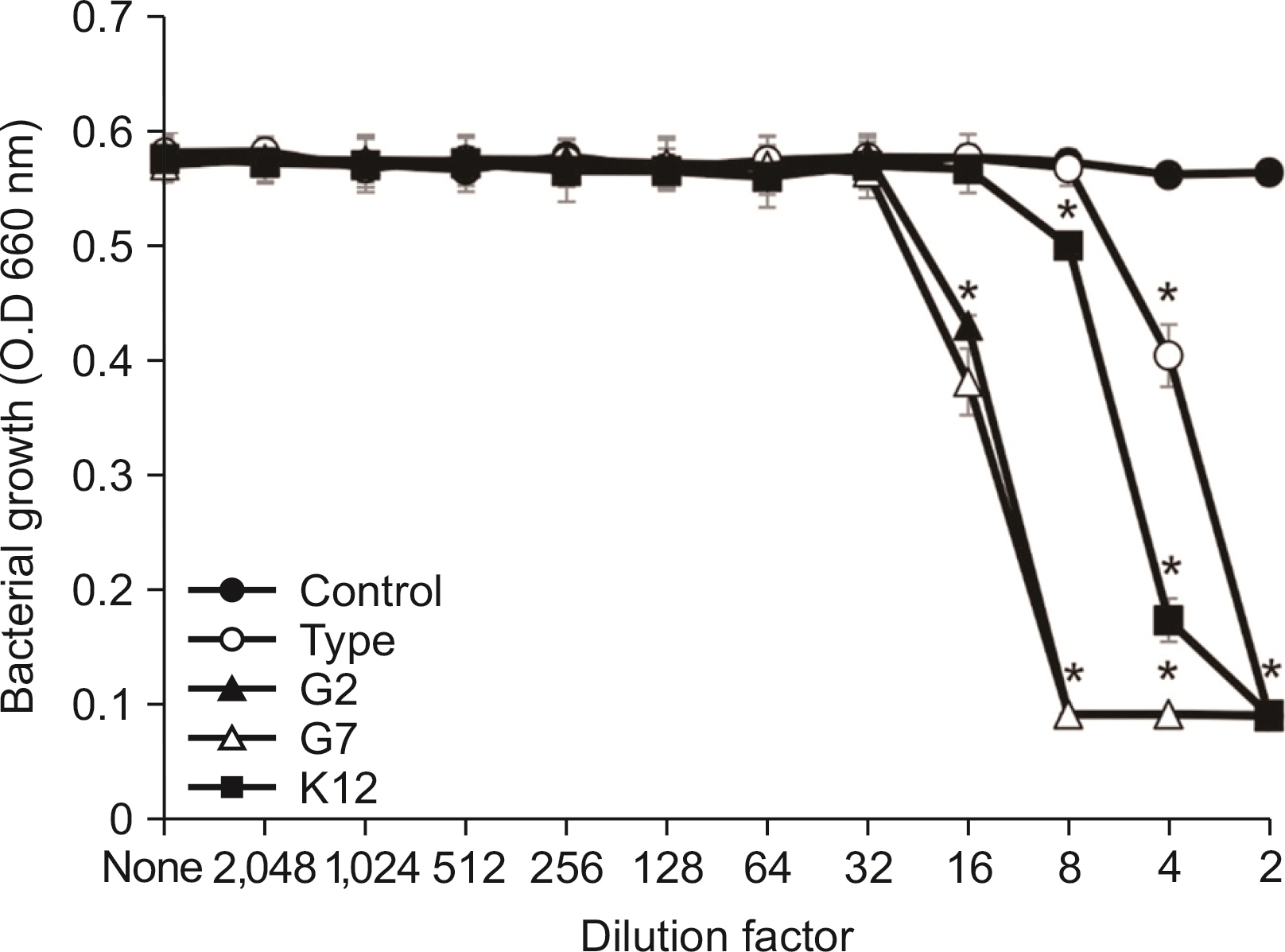
All S. salivarius have antimicrobial activity against Gram-positive bacteria in oral cavity. When comparing antimicrobial activity, S. salivarius G2 and G7 as isolated strain showed stronger antimicrobial activity against Gram-positive microbe than type K12 strain.
Conclusions
S. salivarius G2 and G7 have strong antimicrobial activity and may be prevent oral disease by Gram-positive bacteria in oral cavity.
Keyword
Figure
Reference
-
References
1. Socransky SS, Haffajee AD. 2002; Dental biofilms: difficult therapeutic targets. Periodontol 2000. 28:12–55. DOI: 10.1034/j.1600-0757.2002.280102.x. PMID: 12013340.2. Marsh PD. 1994; Microbial ecology of dental plaque and its significance in health and disease. Adv Dent Res. 8:263–271. DOI: 10.1177/08959374940080022001. PMID: 7865085.3. Howell A, Stephan RM, Paul F. 1962; Prevalence of Actin-omyces israelii, A. naeslundii, Bacterionema matruchotii, and Candida albicans in selected areas of the oral cavity and saliva. J Dent Res. 41:1050–1059. DOI: 10.1177/00220345620410050701. PMID: 13955156.4. Loesche WJ, Syed SA. 1978; Bacteriology of human experimental gingivitis: effect of plaque and gingivitis score. Infect Immun. 21:830–839. DOI: 10.1128/iai.21.3.830-839.1978. PMID: 711337. PMCID: PMC422072.5. Conrads G, de Soet JJ, Song L, Henne K, Sztajer H, Wagner-Dobler I, et al. 2014; Comparing the cariogenic species Streptococcus sobrinus and S. mutans on whole genome level. J Oral Microbiol. 6:26189. DOI: 10.3402/jom.v6.26189. PMID: 25475081. PMCID: PMC4256546.6. Lee SH, Choi BK, Kim YJ. 2012; The cariogenic characters of xylitol-resistant and xylitol-sensitive Streptococcus mutans in biofilm formation with salivary bacteria. Arch Oral Biol. 57:697–703. DOI: 10.1016/j.archoralbio.2011.12.001. PMID: 22218085.7. Dahlen G. 1993; Role of suspected periodontopathogens in microbiological monitoring of periodontitis. Adv Dent Res. 7:163–174. DOI: 10.1177/08959374930070020701. PMID: 8260004.8. Rams TE, Feik D, Mortensen JE, Degener JE, Winkelhoff AJ. 2013; Antibiotic susceptibility of periodontal Enterococcus faecalis. J Periodontol. 84:1026–1033. DOI: 10.1902/jop.2012.120050. PMID: 23106507.9. Delorme C, Abraham AL, Renault P, Guedon E. 2015; Genomics of Streptococcus salivarius, a major human commensal. Infect Genet Evol. 33:381–392. DOI: 10.1016/j.meegid.2014.10.001. PMID: 25311532.10. Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. 2005; Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 43:5721–5732. DOI: 10.1128/JCM.43.11.5721-5732.2005. PMID: 16272510. PMCID: PMC1287824.11. Wescombe PA, Burton JP, Cadieux PA, Klesse NA, Hyink O, Heng NC, et al. 2006; Megaplasmids encode differing combinations of lantibiotics in Streptococcus salivarius. Antonie Van Leeuwenhoek. 90:269–280. DOI: 10.1007/s10482-006-9081-y. PMID: 16871420.12. Li X, Fields FR, Ho M, Marshall-Hudson A, Gross R, Casser ME, et al. 2021; Safety assessment of Streptococcus salivarius DB-B5 as a probiotic candidate for oral health. Food Chem Toxicol. 153:112277. DOI: 10.1016/j.fct.2021.112277. PMID: 34004226.13. Hale JDF, Jain R, Wescombe PA, Burton JP, Simon RR, Tagg JR. 2022; Safety assessment of Streptococcus salivarius M18 a probiotic for oral health. Benef Microbes. 13:47–60. DOI: 10.3920/BM2021.0107. PMID: 35098909.14. Laws GL, Hale JDF, Kemp RA. 2021; Human Systemic Immune Response to Ingestion of the Oral Probiotic Streptococcus salivarius BLIS K12. Probiotics Antimicrob Proteins. 13:1521–1529. DOI: 10.1007/s12602-021-09822-3. PMID: 34282568.15. Hyink O, Wescombe PA, Upton M, Ragland N, Burton JP, Tagg JR. 2007; Salivaricin A2 and the novel lantibiotic salivaricin B are encoded at adjacent loci on a 190-kilobase transmissible megaplasmid in the oral probiotic strain Streptococcus salivarius K12. Appl Environ Microbiol. 73:1107–1113. DOI: 10.1128/AEM.02265-06. PMID: 17194838. PMCID: PMC1828679.16. Wescombe PA, Upton M, Dierksen KP, Ragland NL, Sivabalan S, Wirawan RE, et al. 2006; Production of the lantibiotic salivaricin A and its variants by oral streptococci and use of a specific induction assay to detect their presence in human saliva. Appl Environ Microbiol. 72:1459–1466. DOI: 10.1128/AEM.72.2.1459-1466.2006. PMID: 16461700. PMCID: PMC1392966.17. Wescombe PA, Upton M, Renault P, Wirawan RE, Power D, Burton JP, Chilcott CN, Tagg JR. 2011; Salivaricin 9, a new lantibiotic produced by Streptococcus salivarius. Microbiology. 157:1290–1299. DOI: 10.1099/mic.0.044719-0. PMID: 21310787.18. Wescombe PA, Dyet KH, Dierksen KP, Power DA, Jack RW, Burton JP, et al. 2012; Salivaricin G32, a Homolog of the Prototype Streptococcus pyogenes Nisin-Like Lantibiotic SA-FF22, Produced by the Commensal Species Streptococcus salivarius. Int J Microbiol. 2012:738503. DOI: 10.1155/2012/738503. PMID: 22567013. PMCID: PMC3332205.19. Hecht DW, Citron DM, Cox M, Jacobus N, Jenkins SG, Onderdonk A, et al. 2007. Methods for antimicrobial susceptibility testing of anaerobic bacteria; Approved standard-Seventh edition (M11-A7). CLSI document. Clinical and Laboratory Standards Institute;Wayne, PA: p. 47.20. Meurman JH, Stamatova I. 2007; Probiotics: contributions to oral health. Oral Dis. 13:443–451. DOI: 10.1111/j.1601-0825.2007.01386.x. PMID: 17714346.21. Kim YJ, Lee SH. 2016; Inhibitory Effect of Lactococcus lactis HY 449 on Cariogenic Biofilm. J Microbiol Biotechnol. 26:1829–1835. DOI: 10.4014/jmb.1604.04008. PMID: 27435538.22. Barbour A, Tagg J, Abou-Zied K, Philip K. 2016; New insights into the mode of action of the lantibiotic salivaricin B. Sci Rep. 6:31749. DOI: 10.1038/srep31749. PMID: 27526944. PMCID: PMC4985645.23. Barbour A, Philip K. 2014; Variable characteristics of bacteriocin-producing Streptococcus salivarius strains isolated from Malaysian subjects. PLoS One. 9:e100541. DOI: 10.1371/journal.pone.0100541. PMID: 24941127. PMCID: PMC4062538.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Characteristics of antimicrobial activity of Streptococcus salivarius K12 by culture condition
- Identification of Antimicrobial Peptide Hexamers against Oral Pathogens through Rapid Screening of a Synthetic Combinatorial Peptide Library
- Antimicrobial Activity of Oleanolic Acid, Ursolic Acid, and Sophoraflavanone G against Periodontopathogens
- Antimicrobial effects of green tea extract-containing dentifrice
- Antimicrobial Effect of Acanthopanax sessiliflorum Fruit Extracts against Selected Oral Bacteria