J Surg Ultrasound.
2021 Nov;8(2):41-47. 10.46268/jsu.2021.8.2.41.
Clinical Value of Axillary Ultrasonography in Breast Cancer with Lymph Node Metastases
- Affiliations
-
- 1Division of Breast and Endocrine Surgery, Hallym University Sacred Heart Hospital, Anyang, Korea
- 2Department of Surgery, Hallym University Dongtan Sacred Heart Hospital, Hwaseong, Korea
- 3Department of Radiology, Hallym University Sacred Heart Hospital, Anyang, Korea
- KMID: 2525881
- DOI: http://doi.org/10.46268/jsu.2021.8.2.41
Abstract
- Background
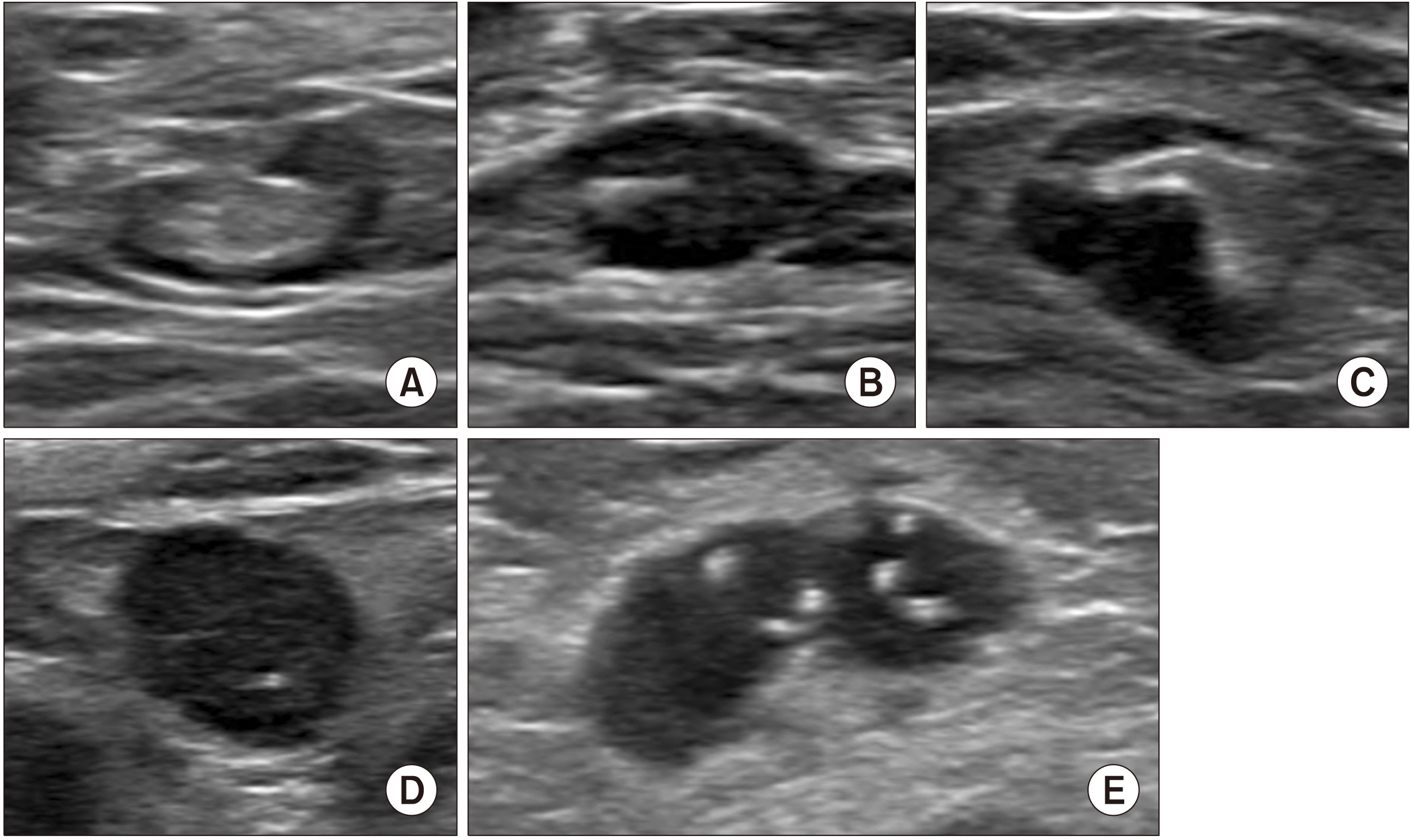
Axillary ultrasonography is a non-invasive and sensitive method used in the evaluation of breast cancer. We sought to evaluate the value of axillary ultrasonography in the nodal staging of breast cancer patients with axillary lymph node metastases.
Methods
From a retrospective database, we reviewed the electronic medical records of breast cancer patients with axillary lymph node metastases who underwent curative surgery between 2003 and 2020. We collected the relevant clinicopathological data and ultrasonographic images. We performed a binary logistic regression analysis to evaluate the factors associated with a high nodal stage.
Results
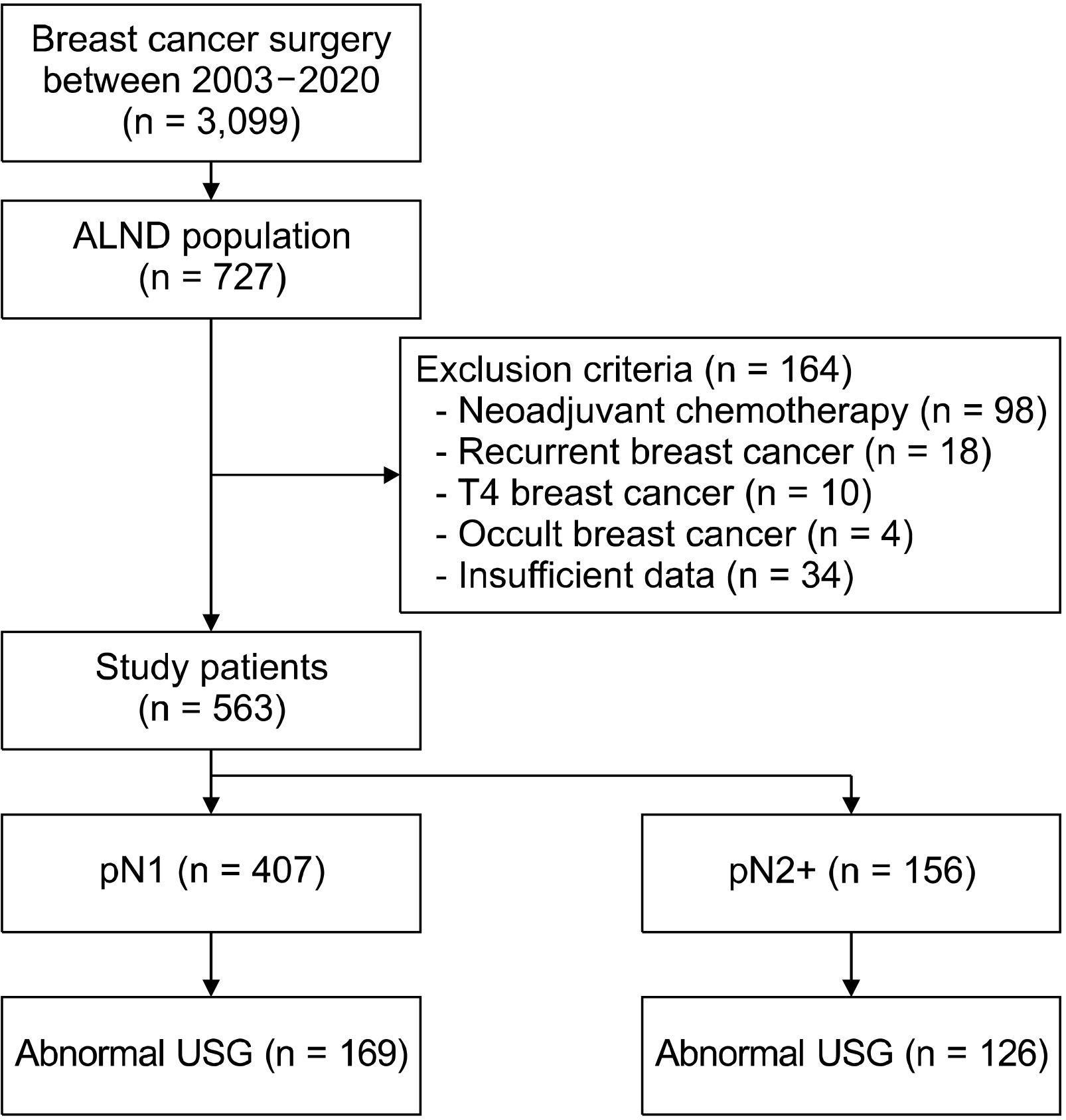
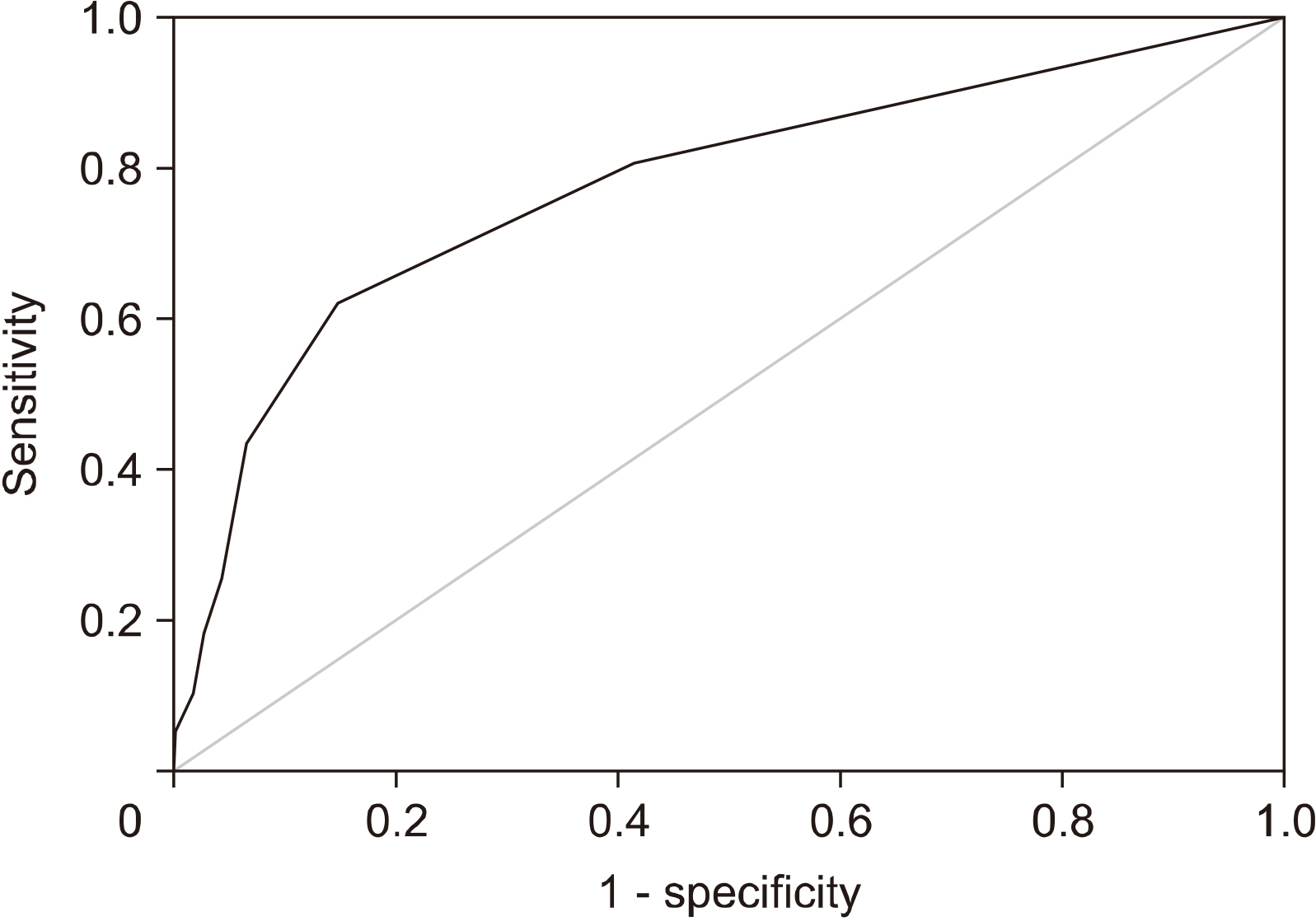
A total of 563 patients were included. Total mastectomy, larger tumor size, abnormal axillary ultrasonography, high histologic grade, lymphatic invasion, hormonal receptor negativity, and HER2 receptor positivity were associated with a pN2 or higher nodal stage. A receiver-operator curve analysis revealed that two or more abnormal lymph nodes seen on axillary ultrasonography identified a high nodal stage with a sensitivity of 62.2% and a specificity of 85.3%. Multivariate analysis revealed that patient age less than 50, lymphatic invasion, two or more abnormal lymph nodes, and hilar effacement were independent predictive factors for the high nodal stage.
Conclusion
In patients with two or more abnormal lymph nodes on axillary ultrasonography, upfront axillary lymph node dissection or neoadjuvant chemotherapy is preferred. Our findings highlight the importance of axillary ultrasonography in the nodal staging of breast cancer.
Keyword
Figure
Reference
-
1. Mansel RE, Fallowfield L, Kissin M, Goyal A, Newcombe RG, Dixon JM, et al. 2006; Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC trial. J Natl Cancer Inst. 98:599–609. DOI: 10.1093/jnci/djj158. PMID: 16670385.
Article2. Castelo M, Hu SY, Dossa F, Acuna SA, Scheer AS. 2020; Comparing observation, axillary radiotherapy, and completion axillary lymph node dissection for management of axilla in breast cancer in patients with positive sentinel nodes: a systematic review. Ann Surg Oncol. 27:2664–76. DOI: 10.1245/s10434-020-08225-y. PMID: 32020394.
Article3. Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR, et al. 2017; Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA. 318:918–26. DOI: 10.1001/jama.2017.11470. PMID: 28898379. PMCID: PMC5672806.
Article4. Elmore LC, Appleton CM, Zhou G, Margenthaler JA. 2013; Axillary ultrasound in patients with clinically node-negative breast cancer: which features are predictive of disease? J Surg Res. 184:234–40. DOI: 10.1016/j.jss.2013.03.068. PMID: 23664535.
Article5. Chang JM, Leung JWT, Moy L, Ha SM, Moon WK. 2020; Axillary nodal evaluation in breast cancer: state of the art. Radiology. 295:500–15. DOI: 10.1148/radiol.2020192534. PMID: 32315268.
Article6. Youn HJ, Ahn HR, Kang SY, Jung SH. 2020; Ultrasonography for Staging axillary lymph node in breast cancer patients. J Surg Ultrasound. 7:1–6. DOI: 10.46268/jsu.2020.7.1.1.
Article7. Choi YJ, Ko EY, Han BK, Shin JH, Kang SS, Hahn SY. 2009; High-resolution ultrasonographic features of axillary lymph node metastasis in patients with breast cancer. Breast. 18:119–22. DOI: 10.1016/j.breast.2009.02.004. PMID: 19297159.
Article8. Farrell TP, Adams NC, Stenson M, Carroll PA, Griffin M, Connolly EM, et al. 2015; The Z0011 trial: is this the end of axillary ultrasound in the pre-operative assessment of breast cancer patients? Eur Radiol. 25:2682–7. DOI: 10.1007/s00330-015-3683-6. PMID: 25740803.
Article9. Angarita S, Ye L, Rünger D, Hadaya J, Baker JL, Dawson N, et al. 2021; Assessingthe burden of nodal disease for breast cancer patients with clinically positive nodes: hope for more limited axillary surgery. Ann Surg Oncol. 28:2609–18. DOI: 10.1245/s10434-020-09228-5. PMID: 33084993.
Article10. Lim GH, Upadhyaya VS, Acosta HA, Lim JMA, Allen JC Jr, Leong LCH. 2018; Preoperative predictors of high and low axillary nodal burden in Z0011 eligible breast cancer patients with a positive lymph node needle biopsy result. Eur J Surg Oncol. 44:945–50. DOI: 10.1016/j.ejso.2018.04.003. PMID: 29705286.
Article11. Ahn SK, Kim MK, Kim J, Lee E, Yoo TK, Lee HB, et al. 2017; Can we skip intraoperative evaluation of sentinel lymph nodes? Nomogram predicting involvement of three or more axillary lymph nodes before breast cancer surgery. Cancer Res Treat. 49:1088–96. DOI: 10.4143/crt.2016.473. PMID: 28161935. PMCID: PMC5654155.
Article12. Gentilini O, Veronesi U. 2012; Abandoning sentinel lymph node biopsy in early breast cancer? A new trial in progress at the European Institute of Oncology of Milan (SOUND: Sentinel node vs Observation after axillary UltraSouND). Breast. 21:678–81. DOI: 10.1016/j.breast.2012.06.013. PMID: 22835916.13. Pilewskie M, Jochelson M, Gooch JC, Patil S, Stempel M, Morrow M. 2016; Is preoperative axillary imaging beneficial in identifying clinically node-negative patients requiring axillary lymph node dissection? J Am Coll Surg. 222:138–45. DOI: 10.1016/j.jamcollsurg.2015.11.013. PMID: 26711795. PMCID: PMC4729648.
Article14. Puri S, Sharma N, Newcombe RG, Kaushik M, Al-Attar M, Pascaline S, et al. 2018; Axillary tumour burden in women with one abnormal node on ultrasound compared to women with multiple abnormal nodes. Clin Radiol. 73:391–5. DOI: 10.1016/j.crad.2017.12.014. PMID: 29352595.
Article15. Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. 2013; Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 310:1455–61. DOI: 10.1001/jama.2013.278932. PMID: 24101169. PMCID: PMC4075763.
Article16. Pilewskie M, Zabor EC, Mamtani A, Barrio AV, Stempel M, Morrow M. 2017; The optimal treatment plan to avoid axillary lymph node dissection in early-stage breast cancer patients differs by surgical strategy and tumor subtype. Ann Surg Oncol. 24:3527–33. DOI: 10.1245/s10434-017-6016-y. PMID: 28762114. PMCID: PMC5697709.
Article17. Schneeweiss A, Chia S, Hickish T, Harvey V, Eniu A, Hegg R, et al. 2013; Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol. 24:2278–84. DOI: 10.1093/annonc/mdt182. PMID: 23704196.
Article18. van der Noordaa MEM, van Duijnhoven FH, Straver ME, Groen EJ, Stokkel M, Loo CE, et al. 2018; Major reduction in axillary lymph node dissections after neoadjuvant systemic therapy for node-positive breast cancer by combining PET/CT and the MARI procedure. Ann Surg Oncol. 25:1512–20. DOI: 10.1245/s10434-018-6404-y. PMID: 29511992.
Article19. Kim WH, Kim HJ, Jung JH, Park HY, Lee J, Kim WW, et al. 2018; Ultrasound-guided restaging and localization of axillary lymph nodes after neoadjuvant chemotherapy for guidance of axillary surgery in breast cancer patients: experience with activated charcoal. Ann Surg Oncol. 25:494–500. DOI: 10.1245/s10434-017-6250-3. PMID: 29134374.
Article20. Kim WH, Kim HJ, Kim SH, Jung JH, Park HY, Lee J, et al. 2019; Ultrasound-guided dual-localization for axillary nodes before and after neoadjuvant chemotherapy with clip and activated charcoal in breast cancer patients: a feasibility study. BMC Cancer. 859. DOI: 10.1186/s12885-019-6095-1. PMID: 31470821. PMCID: PMC6716853.
Article21. National Comprehensive Cancer Network. 2021. NCCN clinical practice guidelines in oncology: Breast Cancer (Version 8, 2021). Available from: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. cited 2021 Nov 24.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ultrasonography for Staging Axillary Lymph Node in Breast Cancer Patients
- Incidence of Axillary Lymph Node Metastases in T1 Breast Cancer
- Incidence of Axillary Lymph Node Metastases in T1 Breast Cancer
- Clinical Significance of Rotter's Nodes in Patients with Breast Carcinomas
- A Recurrence of Ovarian Carcinoma Presenting as Only Axillary Lymphatic Metastasis: A Case Report