Anesth Pain Med.
2021 Oct;16(4):360-367. 10.17085/apm.21001.
Ability of dynamic preload indices to predict fluid responsiveness in a high femoral-to-radial arterial pressure gradient: a retrospective study
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, National Health Insurance Service Ilsan Hospital, Goyang, Korea
- 2Department of Anesthesiology and Pain Medicine, Yonsei University College of Medicine, Seoul, Korea
- 3Department of Research Affairs, Biostatistics Collaboration Unit, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2524448
- DOI: http://doi.org/10.17085/apm.21001
Abstract
- Background
Dynamic preload indices may predict fluid responsiveness in end-stage liver disease. However, their usefulness in patients with altered vascular compliance is uncertain. This study is the first to evaluate whether dynamic indices can reliably predict fluid responsiveness in patients undergoing liver transplantation with a high femoral-to-radial arterial pressure gradient (PG).
Methods
80 liver transplant recipients were retrospectively categorized as having a normal (n = 56) or high (n = 24, difference in systolic pressure ≥ 10 mmHg and/or mean pressure ≥ 5 mmHg) femoral-to-radial arterial PG, measured immediately after radial and femoral arterial cannulation. The ability of dynamic preload indices (stroke volume variation, pulse pressure variation [PPV], pleth variability index) to predict fluid responsiveness was assessed before the surgery. Fluid replacement of 500 ml of crystalloid solution was performed over 15 min. Fluid responsiveness was defined as ≥ 15% increase in the stroke volume index. The area under the receiver-operating characteristic curve (AUC) indicated the prediction of fluid responsiveness.
Results
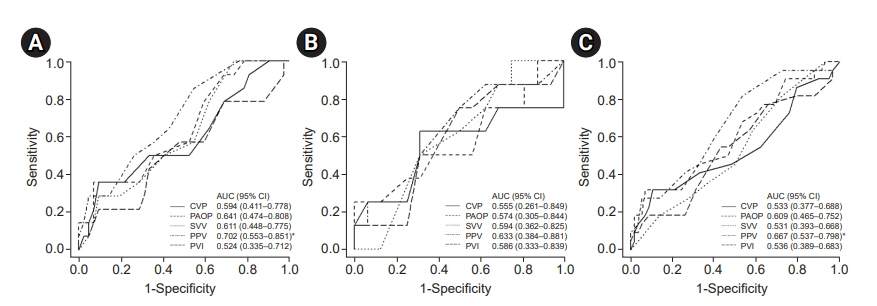
Fourteen patients in the normal, and eight in the high PG group were fluid responders. The AUCs for PPV in the normal, high PG groups and total patients were 0.702 (95% confidence interval [CI] 0.553–0.851, P = 0.008), 0.633 (95% CI 0.384–0.881, P = 0.295) and 0.667 (95% CI 0.537–0.798, P = 0.012), respectively. No other index predicted fluid responsiveness.
Conclusion
PPV can be used as a dynamic index of fluid responsiveness in patients with end-stage liver disease but not in patients with altered vascular compliance.
Figure
Reference
-
1. Izzy M, VanWagner LB, Lin G, Altieri M, Findlay JY, Oh JK, et al. Redefining cirrhotic cardiomyopathy for the modern era. Hepatology. 2020; 71:334–45.2. Schroeder RA, Collins BH, Tuttle-Newhall E, Robertson K, Plotkin J, Johnson LB, et al. Intraoperative fluid management during orthotopic liver transplantation. J Cardiothorac Vasc Anesth. 2004; 18:438–41.3. Gan TJ, Soppitt A, Maroof M, el-Moalem H, Robertson KM, Moretti E, et al. Goal-directed intraoperative fluid administration reduces length of hospital stay after major surgery. Anesthesiology. 2002; 97:820–6.4. Grocott MP, Mythen MG, Gan TJ. Perioperative fluid management and clinical outcomes in adults. Anesth Analg. 2005; 100:1093–106.5. Osman D, Ridel C, Ray P, Monnet X, Anguel N, Richard C, et al. Cardiac filling pressures are not appropriate to predict hemodynamic response to volume challenge. Crit Care Med. 2007; 35:64–8.6. Marik PE, Cavallazzi R, Vasu T, Hirani A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med. 2009; 37:2642–7.7. Preisman S, Kogan S, Berkenstadt H, Perel A. Predicting fluid responsiveness in patients undergoing cardiac surgery: functional haemodynamic parameters including the Respiratory Systolic Variation Test and static preload indicators. Br J Anaesth. 2005; 95:746–55.8. Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest. 2002; 121:2000–8.9. Rex S, Brose S, Metzelder S, Hüneke R, Schälte G, Autschbach R, et al. Prediction of fluid responsiveness in patients during cardiac surgery. Br J Anaesth. 2004; 93:782–8.10. Forget P, Lois F, de Kock M. Goal-directed fluid management based on the pulse oximeter-derived pleth variability index reduces lactate levels and improves fluid management. Anesth Analg. 2010; 111:910–4.11. Wu CY, Cheng YJ, Liu YJ, Wu TT, Chien CT, Chan KC. Predicting stroke volume and arterial pressure fluid responsiveness in liver cirrhosis patients using dynamic preload variables: a prospective study of diagnostic accuracy. Eur J Anaesthesiol. 2016; 33:645–52.12. Shin YH, Ko JS, Gwak MS, Kim GS, Lee JH, Lee SK. Utility of uncalibrated femoral stroke volume variation as a predictor of fluid responsiveness during the anhepatic phase of liver transplantation. Liver Transpl. 2011; 17:53–9.13. Gouvêa G, Diaz R, Auler L, Toledo R, Martinho JM. Evaluation of the pulse pressure variation index as a predictor of fluid responsiveness during orthotopic liver transplantation. Br J Anaesth. 2009; 103:238–43.14. Konur H, Erdogan Kayhan G, Toprak HI, Bucak N, Aydogan MS, Yologlu S, et al. Evaluation of pleth variability index as a predictor of fluid responsiveness during orthotopic liver transplantation. Kaohsiung J Med Sci. 2016; 32:373–80.15. Kim SY, Song Y, Shim JK, Kwak YL. Effect of pulse pressure on the predictability of stroke volume variation for fluid responsiveness in patients with coronary disease. J Crit Care. 2013; 28:318.e1–e7.16. Arnal D, Garutti I, Perez-Peña J, Olmedilla L, Tzenkov IG. Radial to femoral arterial blood pressure differences during liver transplantation. Anaesthesia. 2005; 60:766–71.17. Reuter DA, Felbinger TW, Schmidt C, Kilger E, Goedje O, Lamm P, et al. Stroke volume variations for assessment of cardiac responsiveness to volume loading in mechanically ventilated patients after cardiac surgery. Intensive Care Med. 2002; 28:392–8.18. Zimmermann M, Feibicke T, Keyl C, Prasser C, Moritz S, Graf BM, et al. Accuracy of stroke volume variation compared with pleth variability index to predict fluid responsiveness in mechanically ventilated patients undergoing major surgery. Eur J Anaesthesiol. 2010; 27:555–61.19. Hong SW, Shim JK, Choi YS, Chun DH, Kim JC, Kim BS, et al. Predictors of ineffectual radial arterial pressure monitoring in valvular heart surgery. J Heart Valve Dis. 2009; 18:546–53.20. Khwannimit B, Bhurayanontachai R. Prediction of fluid responsiveness in septic shock patients: comparing stroke volume variation by FloTrac/Vigileo and automated pulse pressure variation. Eur J Anaesthesiol. 2012; 29:64–9.21. Freitas FG, Bafi AT, Nascente AP, Assunção M, Mazza B, Azevedo LC, et al. Predictive value of pulse pressure variation for fluid responsiveness in septic patients using lung-protective ventilation strategies. Br J Anaesth. 2013; 110:402–8.22. Kwon HM, Hwang GS. Cardiovascular dysfunction and liver transplantation. Korean J Anesthesiol. 2018; 71:85–91.23. Ruiz-del-Árbol L, Serradilla R. Cirrhotic cardiomyopathy. World J Gastroenterol. 2015; 21:11502–21.24. Lansdorp B, Lemson J, van Putten MJ, de Keijzer A, van der Hoeven JG, Pickkers P. Dynamic indices do not predict volume responsiveness in routine clinical practice. Br J Anaesth. 2012; 108:395–401.25. Renner J, Scholz J, Bein B. Monitoring fluid therapy. Best Pract Res Clin Anaesthesiol. 2009; 23:159–71.26. Bouchacourt JP, Riva JA, Grignola JC. The increase of vasomotor tone avoids the ability of the dynamic preload indicators to estimate fluid responsiveness. BMC Anesthesiol. 2013; 13:41.27. Hadian M, Severyn DA, Pinsky MR. The effects of vasoactive drugs on pulse pressure and stroke volume variation in postoperative ventilated patients. J Crit Care. 2011; 26:328.e1–e8.28. Lee M, Weinberg L, Pearce B, Scurrah N, Story DA, Pillai P, et al. Agreement between radial and femoral arterial blood pressure measurements during orthotopic liver transplantation. Crit Care Resusc. 2015; 17:101–7.29. Chauhan S, Saxena N, Mehrotra S, Rao BH, Sahu M. Femoral artery pressures are more reliable than radial artery pressures on initiation of cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2000; 14:274–6.30. Manecke GR Jr, Parimucha M, Stratmann G, Wilson WC, Roth DM, Auger WR, et al. Deep hypothermic circulatory arrest and the femoral-to-radial arterial pressure gradient. J Cardiothorac Vasc Anesth. 2004; 18:175–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Monitoring for fluid management: dynamic guides and fluid responsiveness
- Pulse pressure variation and stroke volume variation to predict fluid responsiveness in patients undergoing carotid endarterectomy
- Study on the Validity of Radial Arterial Pressure during Cardiopulmonary Bypass
- Factors Affecting the Difference between Radial and Femoral Arterial Pressure after Cardiopulmonary Bypass in Patients Undergoing Valvular ReplacementFactors Affecting the Difference between Radial and Femoral Arterial Pressure after
- A comparison of systolic and diastolic time variation with pulse pressure variation as a predictor of fluid responsiveness during pediatric liver transplantation