Prog Med Phys.
2021 Dec;32(4):165-171. 10.14316/pmp.2021.32.4.165.
Effect of Total Collimation Width on Relative Electron Density, Effective Atomic Number, and Stopping Power Ratio Acquired by Dual-Layer Dual-Energy Computed Tomography
- Affiliations
-
- 1Department of Radiation Oncology, Seoul National University Hospital, Seoul, Korea
- 2Institute of Radiation Medicine, Seoul National University Medical Research Center, Seoul, Korea
- 3Biomedical Research Institute, Seoul National University Hospital, Seoul, Korea
- 4Interdisciplinary Program in Bioengineering, Seoul National University, Seoul, Korea
- 5Department of Radiation Oncology, Seoul National University College of Medicine, Seoul, Korea
- 6Robotics Research Laboratory for Extreme Environments, Advanced Institute of Convergence Technology, Suwon, Korea
- KMID: 2524164
- DOI: http://doi.org/10.14316/pmp.2021.32.4.165
Abstract
- Purpose
This study aimed to evaluate the effect of collimator width on effective atomic number (EAN), relative electron density (RED), and stopping power ratio (SPR) measured by dual-layer dual-energy computed tomography (DL-DECT).
Methods
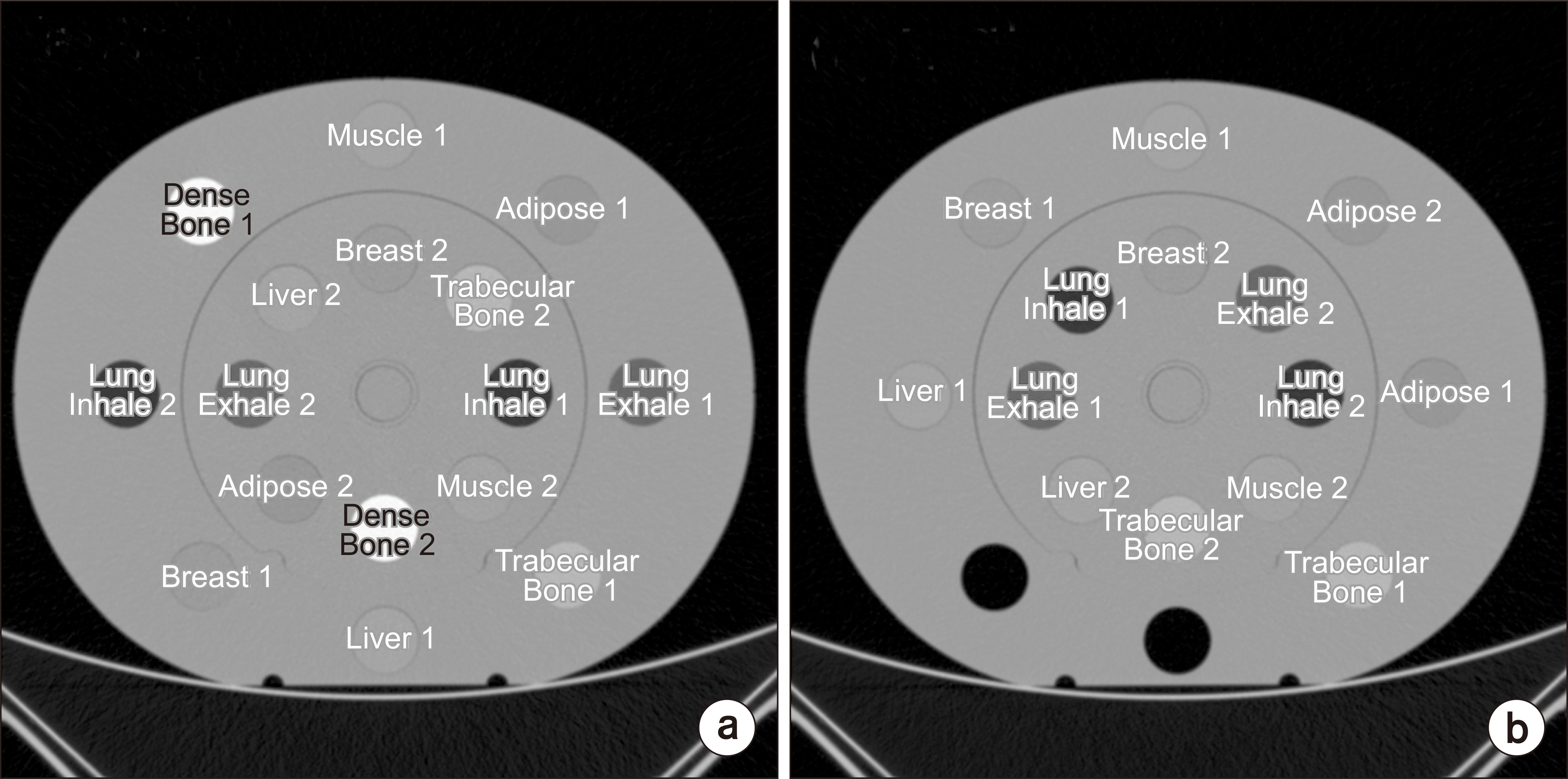
CIRS electron density calibration phantoms with two different arrangements of material plugs were scanned by DL-DECT with two different collimator widths. The first phantom included two dense bone plugs, while the second excluded dense bone plugs. The collimator widths selected were 64 mm×0.625 mm for wider collimators and 16 mm×0.625 mm for narrow collimators. The scanning parameters were 120 kVp, 0.33 second gantry rotation, 3 mm slice thickness, B reconstruction filter, and spectral level 4. An image analysis portal system provided by a computed tomography (CT) manufacturer was used to derive the EAN and RED of the phantoms from the combination of low energy and high energy CT images. The EAN and RED were compared between the images scanned using the two different collimation widths.
Results
The CT images with the wider collimation width generated more severe artifacts, particularly with high-density material (i.e., dense bone). RED and EAN for tissues (excluding lung and bones) with the wider collimation width showed significant relative differences compared to the theoretical value (4.5% for RED and 20.6% for EAN), while those with the narrow collimation width were closer to the theoretical value of each material (2.2% for EAN and 2.3% for RED). Scanning with narrow collimation width increased the accuracy of SPR estimation even with highdensity bone plugs in the phantom.
Conclusions
The effect of CT collimation width on EAN, RED, and SPR measured by DL-DECT was evaluated. In order to improve the accuracy of the measured EAN, RED, and SPR by DL-DECT, CT scanning should be performed using narrow collimation widths.
Keyword
Figure
Reference
-
References
1. Faller FK, Mein S, Ackermann B, Debus J, Stiller W, Mairani A. 2020; Pre-clinical evaluation of dual-layer spectral computed tomography-based stopping power prediction for particle therapy planning at the Heidelberg Ion Beam Therapy Center. Phys Med Biol. 65:095007. DOI: 10.1088/1361-6560/ab735e. PMID: 32028273.
Article2. Jung S, Kim B, Kim JI, Park JM, Choi CH. 2020; Deriving the effective atomic number with a dual-energy image set acquired by the Big Bore CT simulator. J Radiat Prot Res. 45:171–177. DOI: 10.14407/jrpr.2020.45.4.171.
Article3. Hua CH, Shapira N, Merchant TE, Klahr P, Yagil Y. 2018; Accuracy of electron density, effective atomic number, and iodine concentration determination with a dual-layer dual-energy computed tomography system. Med Phys. 45:2486–2497. DOI: 10.1002/mp.12903. PMID: 29624708.
Article4. Ohira S, Washio H, Yagi M, Karino T, Nakamura K, Ueda Y, et al. 2018; Estimation of electron density, effective atomic number and stopping power ratio using dual-layer computed tomography for radiotherapy treatment planning. Phys Med. 56:34–40. DOI: 10.1016/j.ejmp.2018.11.008. PMID: 30527087.
Article5. Mei K, Ehn S, Oechsner M, Kopp FK, Pfeiffer D, Fingerle AA, et al. 2018; Dual-layer spectral computed tomography: measuring relative electron density. Eur Radiol Exp. 2:20. DOI: 10.1186/s41747-018-0051-8. PMID: 30175319. PMCID: PMC6103960.
Article6. Yu L, McCollough CH, Leng S, Kofler JM. 2011; 2011 Joint AAPM/COMP meeting program. Optimization of image acquisition and reconstruction in multi-slice CT. Med Phys. 38(6Pt33):3822. DOI: 10.1118/1.3613393.7. Edward Boas F, Fleischmann D. 2012; CT artifacts: causes and reduction techniques. Imaging Med. 4:229–240. DOI: 10.2217/iim.12.13.8. Almeida IP, Schyns LEJR, Vaniqui A, van der Heyden B, Dedes G, Resch AF, et al. 2018; Monte Carlo proton dose calculations using a radiotherapy specific dual-energy CT scanner for tissue segmentation and range assessment. Phys Med Biol. 63:115008. DOI: 10.1088/1361-6560/aabb60. PMID: 29616662.
Article9. Landry G, Parodi K, Wildberger JE, Verhaegen F. 2013; Deriving concentrations of oxygen and carbon in human tissues using single- and dual-energy CT for ion therapy applications. Phys Med Biol. 58:5029–5048. DOI: 10.1088/0031-9155/58/15/5029. PMID: 23831541.
Article10. Paganetti H. 2012; Range uncertainties in proton therapy and the role of Monte Carlo simulations. Phys Med Biol. 57:R99–R117. DOI: 10.1088/0031-9155/57/11/R99. PMID: 22571913. PMCID: PMC3374500.
Article11. Wohlfahrt P, Möhler C, Hietschold V, Menkel S, Greilich S, Krause M, et al. 2017; Clinical implementation of dual-energy CT for proton treatment planning on pseudomonoenergetic CT scans. Int J Radiat Oncol Biol Phys. 97:427–434. DOI: 10.1016/j.ijrobp.2016.10.022. PMID: 28068248.
Article12. Schaffner B, Pedroni E. 1998; The precision of proton range calculations in proton radiotherapy treatment planning: experimental verification of the relation between CT-HU and proton stopping power. Phys Med Biol. 43:1579–1592. DOI: 10.1088/0031-9155/43/6/016. PMID: 9651027.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Novel Approach for Estimating the Effective Atomic Number Using Dual Energy
- Analysis of Beam Hardening of Modulation Layers for Dual Energy Cone-beam CT
- Dual-Layer Computed Tomography in Cardiovascular Imaging
- Dual-Energy CT: New Horizon in Medical Imaging
- Evaluation of Radiation Dose for Dual Energy CBCT Using Multi-Grid Device