Trends and Risk Factors of Metabolic Syndrome among Korean Adolescents, 2007 to 2018
- Affiliations
-
- 1Department of Pediatrics, Inje University Sanggye Paik Hospital, Inje University College of Medicine, Seoul, Korea
- KMID: 2522727
- DOI: http://doi.org/10.4093/dmj.2020.0185
Abstract
- Background
There is a lack of recent research on the changes in risk factors for metabolic syndrome (MetS) in the Asian pediatric population. We aimed to determine the 12-year trends in the prevalence of MetS and relevant lifestyle factors such as smoking, exercise, and calorie intake among Korean adolescents.
Methods
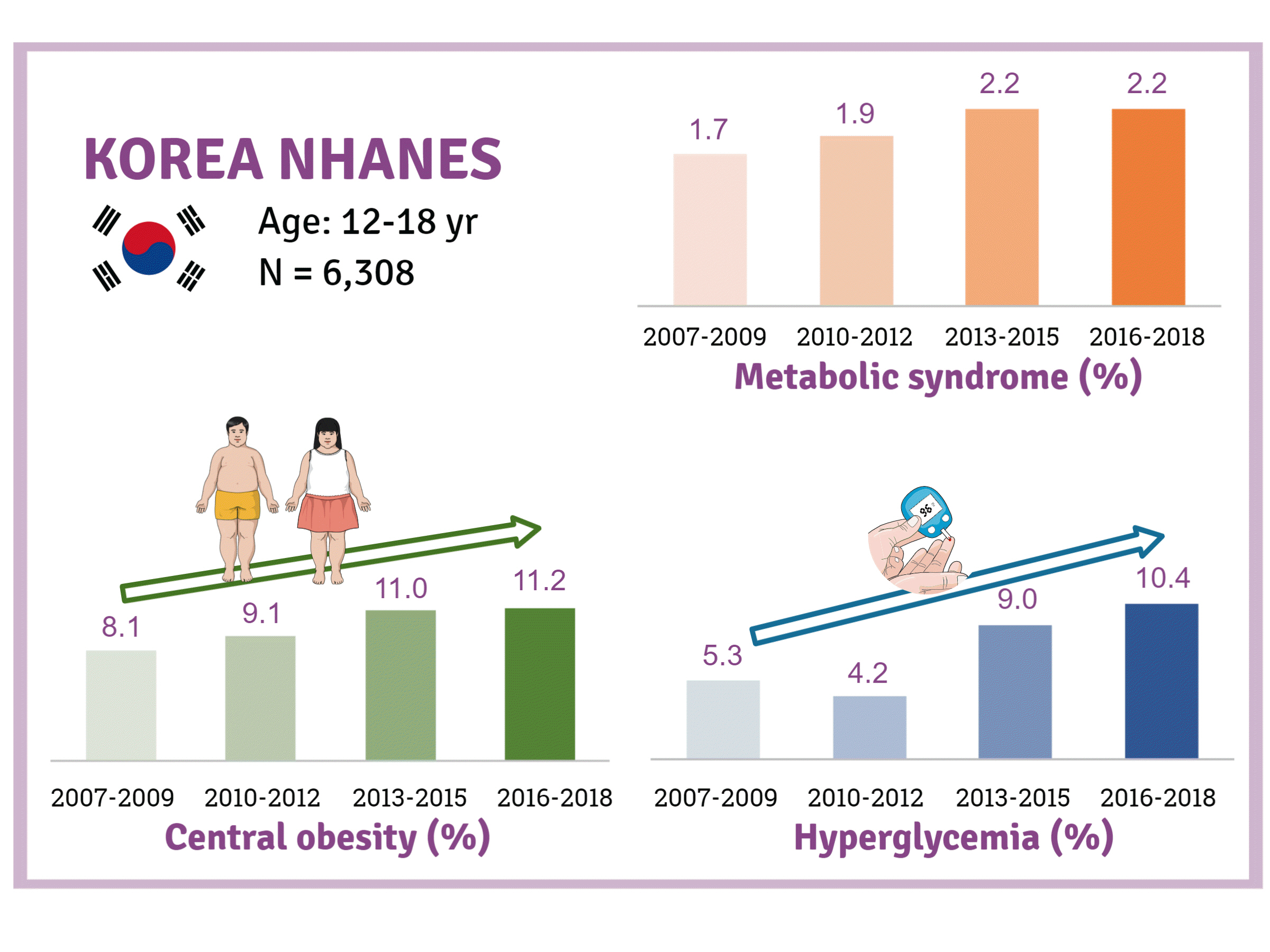
We investigated trends in MetS and lifestyle factors among 6,308 adolescents aged 12 to 18 years from the Korea National Health and Nutrition Examination Survey, 2007 to 2018.
Results
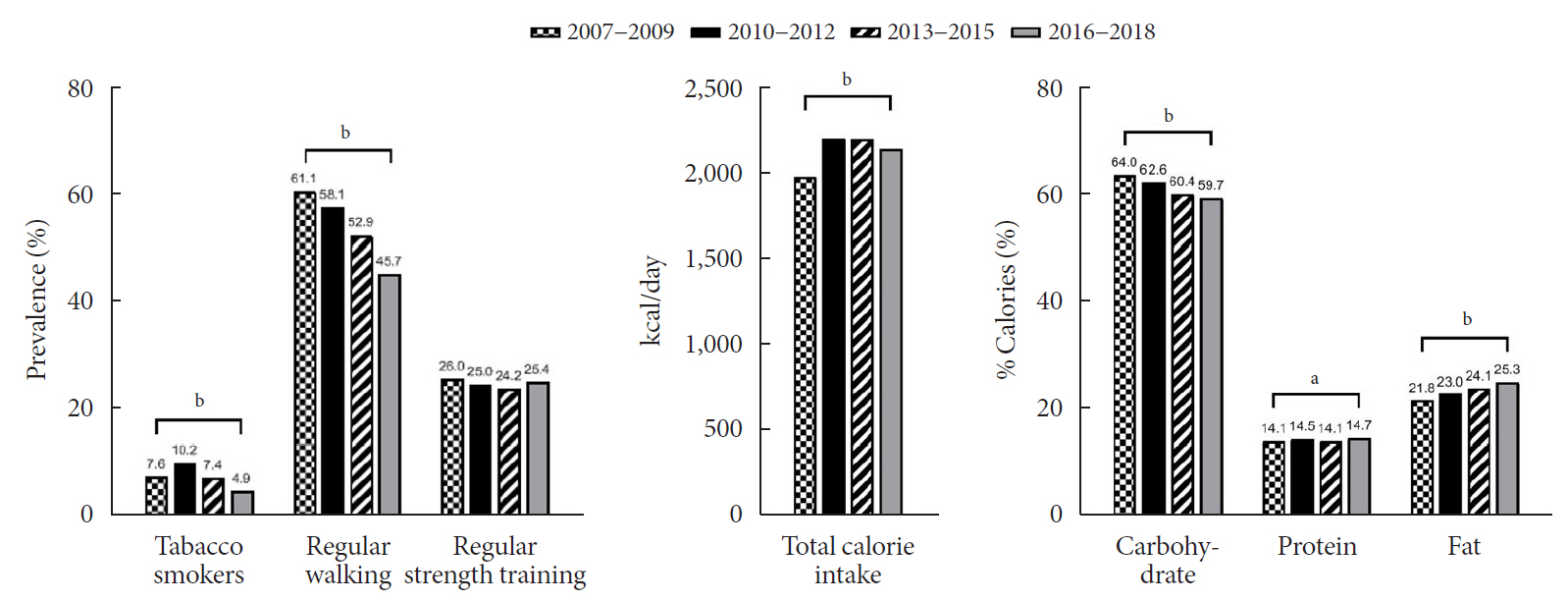
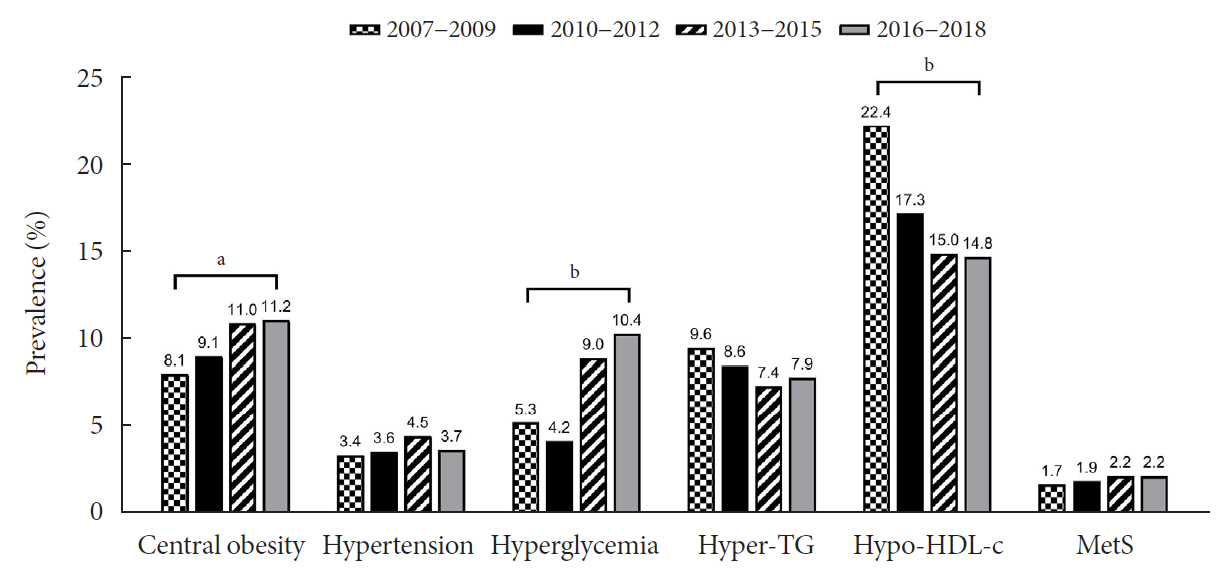
The prevalence of MetS was stable from 2007 to 2018 (1.7% to 2.2%). There were significant increases in the prevalence of central obesity (from 8.1% to 11.2%, P=0.012) and hyperglycemia (from 5.3% to 10.4%, P<0.001) and decreases in hypo-high-density lipoprotein (HDL)-cholesterolemia (from 22.4% to 14.8%, P<0.001). Total calorie intake and calorie intake from fat significantly increased (P<0.001), whereas calorie intake from carbohydrates significantly decreased (P<0.001) during the study period. The proportions of tobacco smokers and regular walkers significantly decreased from 2007 to 2018. After controlling for all covariates, total calorie intake was positively correlated with waist circumference (P<0.05). HDL-cholesterol was negatively associated with carbohydrate consumption (P<0.01) and positively associated with fat consumption (P<0.001). Regular walking and regular strength training were associated with lower waist circumference (P<0.05). Smoking was associated with lower fasting glucose levels (P<0.01).
Conclusion
Although the prevalence rate of MetS is stable among Korean adolescents, the prevalence of central obesity and hyperglycemia has increased greatly in the recent decade. Public education on proper dietary intake and lifestyle modification is required.
Keyword
Figure
Cited by 3 articles
-
Trends and Risk Factors of Metabolic Syndrome among Korean Adolescents, 2007 to 2018 (
Diabetes Metab J 2021;45:880-9)
Dae Jung Kim
Diabetes Metab J. 2022;46(2):349-350. doi: 10.4093/dmj.2021.0353.Trends and Risk Factors of Metabolic Syndrome among Korean Adolescents, 2007 to 2018 (
Diabetes Metab J 2021;45:880-9)
Jiun Chae, Moon Young Seo, Shin-Hye Kim, Mi Jung Park
Diabetes Metab J. 2022;46(2):351-353. doi: 10.4093/dmj.2021.0367.Comprehensive Understanding for Application in Korean Patients with Type 2 Diabetes Mellitus of the Consensus Statement on Carbohydrate-Restricted Diets by Korean Diabetes Association, Korean Society for the Study of Obesity, and Korean Society of Hypertension
Jong Han Choi, Jee-Hyun Kang, Suk Chon
Diabetes Metab J. 2022;46(3):377-390. doi: 10.4093/dmj.2022.0051.
Reference
-
1. Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: definitions and controversies. BMC Med. 2011; 9:48.
Article2. Morrison JA, Friedman LA, Gray-McGuire C. Metabolic syndrome in childhood predicts adult cardiovascular disease 25 years later: the Princeton Lipid Research Clinics Follow-up Study. Pediatrics. 2007; 120:340–5.
Article3. Morrison JA, Friedman LA, Wang P, Glueck CJ. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. J Pediatr. 2008; 152:201–6.
Article4. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017; 390:2627–42.5. Lim S, Jang HC, Park KS, Cho SI, Lee MG, Joung H, et al. Changes in metabolic syndrome in American and Korean youth, 1997-2008. Pediatrics. 2013; 131:e214–22.
Article6. Lee AM, Gurka MJ, DeBoer MD. Trends in metabolic syndrome severity and lifestyle factors among adolescents. Pediatrics. 2016; 137:e20153177.
Article7. Barzin M, Hosseinpanah F, Saber H, Sarbakhsh P, Nakhoda K, Azizi F. Gender differences time trends for metabolic syndrome and its components among Tehranian children and adolescents. Cholesterol. 2012; 2012:804643.
Article8. Park MJ, Boston BA, Oh M, Jee SH. Prevalence and trends of metabolic syndrome among Korean adolescents: from the Korean NHANES survey, 1998-2005. J Pediatr. 2009; 155:529–34.
Article9. International Diabetes Federation: The IDF consensus definition of the metabolic syndrome in children and adolescents. Available from: https://www.idf.org/e-library/consensusstatements/61-idf-consensus-definition-of-metabolic-syndrome-in-children-and-adolescents.html (cited 2021 May 7).10. Lee SY, Park HS, Kim DJ, Han JH, Kim SM, Cho GJ, et al. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res Clin Pract. 2007; 75:72–80.
Article11. Zhu Y, Zheng H, Zou Z, Jing J, Ma Y, Wang H, et al. Metabolic syndrome and related factors in chinese children and adolescents: analysis from a Chinese national study. J Atheroscler Thromb. 2020; 27:534–44.
Article12. Ford ES, Li C, Zhao G, Pearson WS, Mokdad AH. Prevalence of the metabolic syndrome among U.S. adolescents using the definition from the International Diabetes Federation. Diabetes Care. 2008; 31:587–9.
Article13. Park J, Hilmers DC, Mendoza JA, Stuff JE, Liu Y, Nicklas TA. Prevalence of metabolic syndrome and obesity in adolescents aged 12 to 19 years: comparison between the United States and Korea. J Korean Med Sci. 2010; 25:75–82.
Article14. Kelishadi R, Heshmat R, Farzadfar F, Esmaeil Motlag M, Bahreynian M, Safiri S, et al. Prevalence of cardio-metabolic risk factors in a nationally representative sample of Iranian adolescents: the CASPIAN-III Study. J Cardiovasc Thorac Res. 2017; 9:12–20.
Article15. Kelishadi R, Hovsepian S, Djalalinia S, Jamshidi F, Qorbani M. A systematic review on the prevalence of metabolic syndrome in Iranian children and adolescents. J Res Med Sci. 2016; 21:90.
Article16. Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014; 384:766–81.17. Liang YJ, Xi B, Song AQ, Liu JX, Mi J. Trends in general and abdominal obesity among Chinese children and adolescents 1993-2009. Pediatr Obes. 2012; 7:355–64.
Article18. Zhang YX, Wang SR, Chen M, Cheng Y. Recent trends in body mass index and waist circumference among children and adolescents in Shandong China. J Trop Pediatr. 2017; 63:461–7.
Article19. Casagrande D, Waib PH, Sgarbi JA. Increase in the prevalence of abdominal obesity in Brazilian school children (2000-2015). Int J Pediatr Adolesc Med. 2017; 4:133–7.
Article20. Hardy LL, Mihrshahi S, Gale J, Drayton BA, Bauman A, Mitchell J. 30-Year trends in overweight, obesity and waist-to-height ratio by socioeconomic status in Australian children, 1985 to 2015. Int J Obes (Lond). 2017; 41:76–82.
Article21. Suder A, Gomula A, Koziel S. Central overweight and obesity in Polish schoolchildren aged 7-18 years: secular changes of waist circumference between 1966 and 2012. Eur J Pediatr. 2017; 176:909–16.22. Xi B, Mi J, Zhao M, Zhang T, Jia C, Li J, et al. Trends in abdominal obesity among U.S. children and adolescents. Pediatrics. 2014; 134:e334–9.
Article23. Grigorakis DA, Georgoulis M, Psarra G, Tambalis KD, Panagiotakos DB, Sidossis LS. Prevalence and lifestyle determinants of central obesity in children. Eur J Nutr. 2016; 55:1923–31.
Article24. El-Kassas G, Ziade F. Exploration of the risk factors of generalized and central obesity among adolescents in North Lebanon. J Environ Public Health. 2017; 2017:2879075.
Article25. Jenkins GP, Evenson KR, Herring AH, Hales D, Stevens J. Cardiometabolic correlates of physical activity and sedentary patterns in U.S. youth. Med Sci Sports Exerc. 2017; 49:1826–33.
Article26. LeBlanc AG, Janssen I. Dose-response relationship between physical activity and dyslipidemia in youth. Can J Cardiol. 2010; 26:201–5.
Article27. Whitaker KM, Pettee Gabriel K, Buman MP, Pereira MA, Jacobs DR Jr, Reis JP, et al. Associations of accelerometer-measured sedentary time and physical activity with prospectively assessed cardiometabolic risk factors: the CARDIA study. J Am Heart Assoc. 2019; 8:e010212.
Article28. Heshmat R, Qorbani M, Shahr Babaki AE, Djalalinia S, AtaeiJafari A, Motlagh ME, et al. Joint association of screen time and physical activity with cardiometabolic risk factors in a national sample of Iranian adolescents: the CASPIANIII Study. PLoS One. 2016; 11:e0154502.
Article29. Baran J, Weres A, Czenczek-Lewandowska E, Wyszynska J, Luszczki E, Deren K, et al. Blood lipid profile and body composition in a pediatric population with different levels of physical activity. Lipids Health Dis. 2018; 17:171.
Article30. Mann S, Beedie C, Jimenez A. Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: review, synthesis and recommendations. Sports Med. 2014; 44:211–21.
Article31. Wang Y, Xu D. Effects of aerobic exercise on lipids and lipoproteins. Lipids Health Dis. 2017; 16:132.
Article32. Yanai H, Katsuyama H, Hamasaki H, Abe S, Tada N, Sako A. Effects of dietary fat intake on HDL metabolism. J Clin Med Res. 2015; 7:145–9.
Article33. Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003; 77:1146–55.
Article34. Morton AM, Furtado JD, Mendivil CO, Sacks FM. Dietary unsaturated fat increases HDL metabolic pathways involving apoE favorable to reverse cholesterol transport. JCI Insight. 2019; 4:e124620.
Article35. Desroches S, Paradis ME, Perusse M, Archer WR, Bergeron J, Couture P, et al. Apolipoprotein A-I, A-II, and VLDL-B-100 metabolism in men: comparison of a low-fat diet and a high-monounsaturated fatty acid diet. J Lipid Res. 2004; 45:2331–8.36. Lee AM, Fermin CR, Filipp SL, Gurka MJ, DeBoer MD. Examining trends in prediabetes and its relationship with the metabolic syndrome in US adolescents, 1999-2014. Acta Diabetol. 2017; 54:373–81.
Article37. Umapathi KK, Thavamani A, Al-Kindi S. Prediabetes in children and adolescents in the United States: prevalence estimates and comorbidities: a population analysis. J Pediatr Endocrinol Metab. 2019; 32:187–9.38. Strasser B. Physical activity in obesity and metabolic syndrome. Ann N Y Acad Sci. 2013; 1281:141–59.
Article39. von Frankenberg AD, Marina A, Song X, Callahan HS, Kratz M, Utzschneider KM. A high-fat, high-saturated fat diet decreases insulin sensitivity without changing intra-abdominal fat in weight-stable overweight and obese adults. Eur J Nutr. 2017; 56:431–43.
Article40. Stettler R, Ith M, Acheson KJ, Decombaz J, Boesch C, Tappy L, et al. Interaction between dietary lipids and physical inactivity on insulin sensitivity and on intramyocellular lipids in healthy men. Diabetes Care. 2005; 28:1404–9.
Article41. Morimoto A, Tatsumi Y, Deura K, Mizuno S, Ohno Y, Watanabe S. Impact of cigarette smoking on impaired insulin secretion and insulin resistance in Japanese men: the Saku Study. J Diabetes Investig. 2013; 4:274–80.
Article42. Piatti P, Setola E, Galluccio E, Costa S, Fontana B, Stuccillo M, et al. Smoking is associated with impaired glucose regulation and a decrease in insulin sensitivity and the disposition index in first-degree relatives of type 2 diabetes subjects independently of the presence of metabolic syndrome. Acta Diabetol. 2014; 51:793–9.
Article43. Xu M, Zhou Y, Xu B, Sun J, Wang T, Lu J, et al. Associations of smoking and alcohol consumption with impaired β-cell function in Chinese men. J Diabetes. 2016; 8:434–41.44. Ostgren CJ, Lindblad U, Ranstam J, Melander A, Rastam L; Skaraborg Hypertension and Diabetes Project. Associations between smoking and beta-cell function in a non-hypertensive and non-diabetic population. Skaraborg Hypertension and Diabetes Project. Diabet Med. 2000; 17:445–50.45. Haskins AE, Bertone-Johnson ER, Pekow P, Carbone E, Fortner RT, Chasan-Taber L. Smoking during pregnancy and risk of abnormal glucose tolerance: a prospective cohort study. BMC Pregnancy Childbirth. 2010; 10:55.
Article46. Fa-Binefa M, Clara A, Perez-Fernandez S, Grau M, Degano IR, Marti-Lluch R, et al. Early smoking-onset age and risk of cardiovascular disease and mortality. Prev Med. 2019; 124:17–22.
Article47. Kelishadi R, Noori A, Qorbani M, Rahimzadeh S, Djalalinia S, Shafiee G, et al. Are active and passive smoking associated with cardiometabolic risk factors in adolescents? The CASPIAN-III Study. Paediatr Int Child Health. 2016; 36:181–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Trends and Risk Factors of Metabolic Syndrome among Korean Adolescents, 2007 to 2018 (Diabetes Metab J 2021;45:880-9)
- Therapeutic approaches to obesity and metabolic syndrome in children and adolescents
- Clinical Predictive Factors for Metabolic Syndrome in Obese Children and Adolescents
- Trends and Risk Factors of Metabolic Syndrome among Korean Adolescents, 2007 to 2018 (Diabetes Metab J 2021;45:880-9)
- Prevalence of the Metabolic Syndrome in Korean Children and Adolescents according to the International Diabetes Federation Definition in Children and Adolescents