Neurointervention.
2021 Nov;16(3):232-239. 10.5469/neuroint.2021.00227.
REtrospective Multicenter INdian Study of Derivo Embolization Device (REMIND): Periprocedural Safety
- Affiliations
-
- 1Division of Interventional Neurology, Department of Neurology, Mazumdar Shaw Medical Centre, Narayana Health City, Bangalore, India
- 2Department of Interventional Neuroradiology, Institute of Neurosciences, Kolkata, India
- 3Department of Neurosurgery, King Edward Memorial Hospital and Seth G.S. Medical College, Mumbai, India
- 4Department of Diagnostic and Interventional Radiology, Kovai Medical Centre and Hospital, Coimbatore, India
- 5Department of Neurointerventional Surgery, Artemis Hospital, Gurugram, India
- KMID: 2522037
- DOI: http://doi.org/10.5469/neuroint.2021.00227
Abstract
- Purpose
The treatment of aneurysms with characteristics such as complex morphology, fusiform, blister-like, wide neck, or large size has been revolutionized with the introduction of flow diverters. Though flow diverters have several advantages over coiling, they also have certain important disadvantages such as the lack of immediate protection against rupture, the risk of ischemic stroke, the need for antiplatelet therapy, and long latency for complete effect. The Derivo Embolization Device (DED) is a second-generation self-expanding device that is claimed to be less thrombogenic than conventional devices. We retrospectively evaluated the periprocedural safety and risks associated with the DED across 5 centers in India.
Materials and Methods
This is a multicentric, retrospective, observational study of DED, conducted at 5 high volume endovascular therapy centers in India from May 2018 to June 2020. Periprocedural demographic, clinical, and angiographic data were collected from a retrospective review of patient charts.
Results
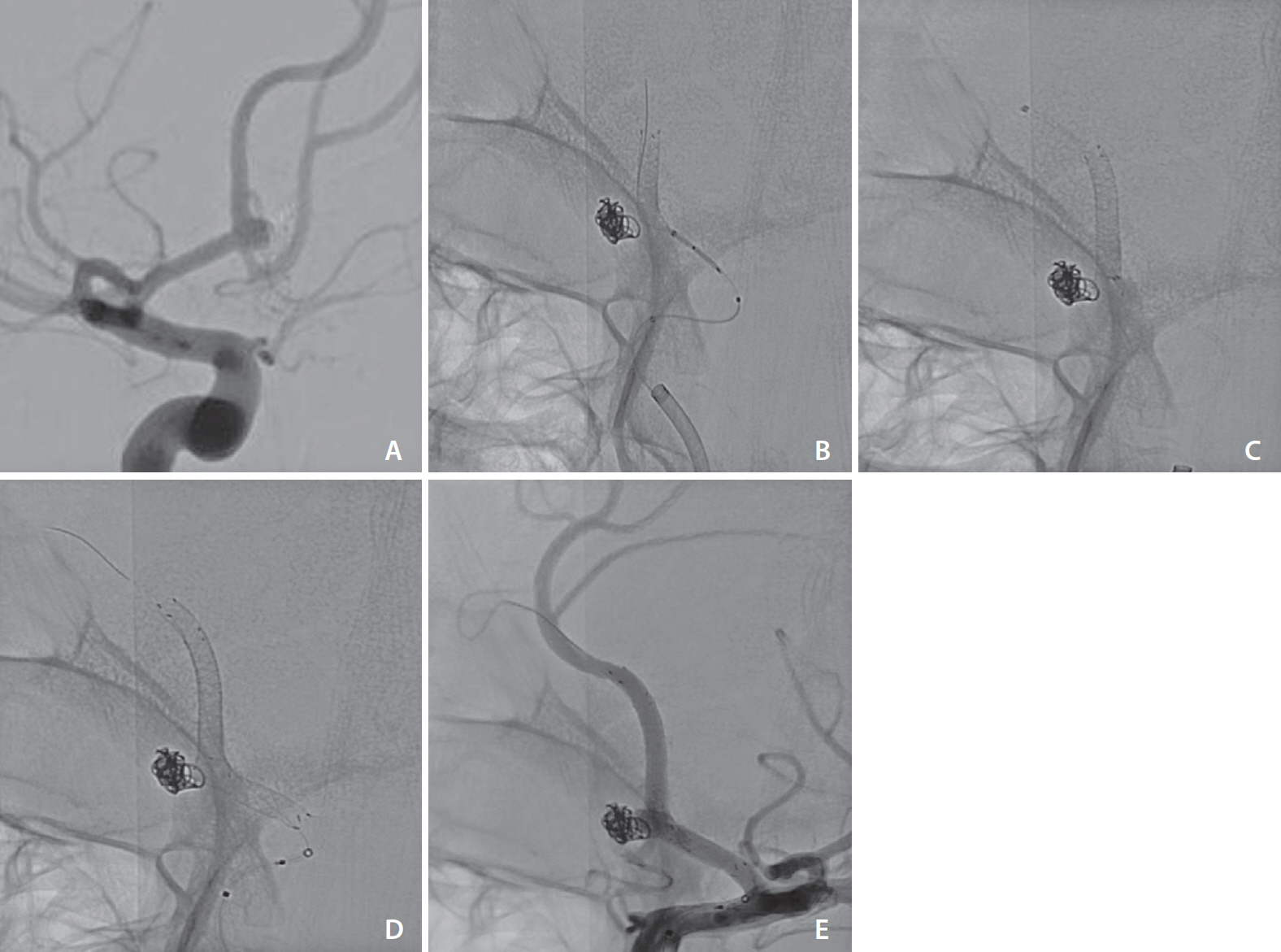
A total of 96 patients, including 56 (58.3%) females, aged between 16–80 years (60±12.7 years) harboring 106 aneurysms were studied. Seven (7.3%) were noted to harbor multiple aneurysms: 6 had 3 aneurysms each, while 1 patient had 5 aneurysms. The following aneurysm characteristics were noted: average size, 9.8±8.2 mm; average neck size, 6.9±8.5 mm; wide-necked (>4 mm), 63 (59.4%); giant (>25 mm), 8 (7.5%); and anterior circulation location, 98 (92.5%). Eighteen (17%) of these were ruptured. Additional balloon angioplasty was performed in 5 (5.2%) patients. Intraprocedural problems were encountered in 3 (3.1%), of which only 1 had clinical implications, the device fish-mouthing with stent thrombosis resulting in a malignant middle cerebral artery territory infarction. The modified Rankin scale at 3 months was worse in 1 patient.
Conclusion
DED is a newer generation flow diverter stent with a low periprocedural complication rate.
Keyword
Figure
Reference
-
1. Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005; 366:809–817.
Article2. Piotin M, Spelle L, Mounayer C, Salles-Rezende MT, Giansante-Abud D, Vanzin-Santos R, et al. Intracranial aneurysms: treatment with bare platinum coils--aneurysm packing, complex coils, and angiographic recurrence. Radiology. 2007; 243:500–508.
Article3. Lylyk P, Miranda C, Ceratto R, Ferrario A, Scrivano E, Luna HR, et al. Curative endovascular reconstruction of cerebral aneurysms with the pipeline embolization device: the Buenos Aires experience. Neurosurgery. 2009; 64:632–642. discussion 642-643; quiz N6.4. Nelson PK, Lylyk P, Szikora I, Wetzel SG, Wanke I, Fiorella D. The pipeline embolization device for the intracranial treatment of aneurysms trial. AJNR Am J Neuroradiol. 2011; 32:34–40.
Article5. Becske T, Kallmes DF, Saatci I, McDougall CG, Szikora I, Lanzino G, et al. Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology. 2013; 267:858–868.
Article6. Trivelato FP, Abud DG, Ulhôa AC, Waihrich ES, Abud TG, Castro Afonso LH, et al. Derivo embolization device for the treatment of intracranial aneurysms. Stroke. 2019; 50:2351–2358.
Article7. Campos JK, Lien BV, Wang AS, Lin LM. Advances in endovascular aneurysm management: coiling and adjunctive devices. Stroke Vasc Neurol. 2020; 5:14–21.
Article8. Akgul E, Onan HB, Akpinar S, Balli HT, Aksungur EH. The DERIVO embolization device in the treatment of intracranial aneurysms: short- and midterm results. World Neurosurg. 2016; 95:229–240.
Article9. Wakhloo AK, Gounis MJ. Revolution in aneurysm treatment: flow diversion to cure aneurysms: a paradigm shift. Neurosurgery. 2014; 61 Suppl 1:111–120.
Article10. Chiu AH, Cheung AK, Wenderoth JD, De Villiers L, Rice H, Phatouros CC, et al. Long-term follow-up results following elective treatment of unruptured intracranial aneurysms with the pipeline embolization device. AJNR Am J Neuroradiol. 2015; 36:1728–1734.
Article11. Kallmes DF, Hanel R, Lopes D, Boccardi E, Bonafé A, Cekirge S, et al. International retrospective study of the pipeline embolization device: a multicenter aneurysm treatment study. AJNR Am J Neuroradiol. 2015; 36:108–115.
Article12. Daglioglu E, Akmangit I, Acik V, Alagoz F, Sayin B, Uckun OM, et al. The experience of the Derivo® embolisation device in intracranial aneurysms. Turk Neurosurg. 2020; 30:30–37.13. Patel A, Miller TR, Shivashankar R, Jindal G, Gandhi D. Early angiographic signs of acute thrombus formation following cerebral aneurysm treatment with the Pipeline embolization device. J Neurointerv Surg. 2017; 9:1125–1130.
Article14. Brinjikji W, Murad MH, Lanzino G, Cloft HJ, Kallmes DF. Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke. 2013; 44:442–447.
Article15. Walcott BP, Stapleton CJ, Choudhri O, Patel AB. Flow diversion for the treatment of intracranial aneurysms. JAMA Neurol. 2016; 73:1002–1008.
Article16. Ley D, Mühl-Benninghaus R, Yilmaz U, Körner H, Cattaneo GFM, Mailänder W, et al. The derivo embolization device, a second-generation flow diverter for the treatment of intracranial aneurysms, evaluated in an elastase-induced aneurysm model. Clin Neuroradiol. 2017; 27:335–343.
Article17. Goertz L, Dorn F, Kraus B, Borggrefe J, Forbrig R, Schlamann M, et al. Improved occlusion rate of intracranial aneurysms treated with the derivo embolization device: one-year clinical and angiographic follow-up in a multicenter study. World Neurosurg. 2019; 126:e1503–e1509.
Article18. Taschner CA, Stracke CP, Dorn F, Kadziolka KB, Kreiser K, Solymosi L, et al. Derivo embolization device in the treatment of unruptured intracranial aneurysms: a prospective multicenter study. J Neurointerv Surg. 2021; 13:541–546.
Article19. Kallmes DF, Brinjikji W, Cekirge S, Fiorella D, Hanel RA, Jabbour P, et al. Safety and efficacy of the Pipeline embolization device for treatment of intracranial aneurysms: a pooled analysis of 3 large studies. J Neurosurg. 2017; 127:775–780.
Article20. Killer-Oberpfalzer M, Kocer N, Griessenauer CJ, Janssen H, Engelhorn T, Holtmannspötter M, et al. European multicenter study for the evaluation of a dual-layer flow-diverting stent for treatment of wide-neck intracranial aneurysms: the European flow-redirection intraluminal device study. AJNR Am J Neuroradiol. 2018; 39:841–847.
Article21. Foa Torres G, Roca F, Noguera A, Godes J, Petrocelli S, Aznar I, et al. Silk flow-diverter stent for the treatment of complex intracranial aneurysms: a one-year follow-up multicenter study. Interv Neuroradiol. 2018; 24:357–362.
Article22. Chalouhi N, Zanaty M, Whiting A, Yang S, Tjoumakaris S, Hasan D, et al. Safety and efficacy of the Pipeline embolization device in 100 small intracranial aneurysms. J Neurosurg. 2015; 122:1498–1502.
Article23. Meyers PM, Coon AL, Kan PT, Wakhloo AK, Hanel RA. SCENT trial. Stroke. 2019; 50:1473–1479.
Article24. Pierot L, Spelle L, Berge J, Januel AC, Herbreteau D, Aggour M, et al. SAFE study (Safety and efficacy Analysis of FRED Embolic device in aneurysm treatment): 1-year clinical and anatomical results. J Neurointerv Surg. 2019; 11:184–189.
Article25. Berge J, Biondi A, Machi P, Brunel H, Pierot L, Gabrillargues J, et al. Flow-diverter silk stent for the treatment of intracranial aneurysms: 1-year follow-up in a multicenter study. AJNR Am J Neuroradiol. 2012; 33:1150–1155.
Article26. Bhatia KD, Kortman H, Orru E, Klostranec JM, Pereira VM, Krings T. Periprocedural complications of second-generation flow diverter treatment using Pipeline Flex for unruptured intracranial aneurysms: a systematic review and meta-analysis. J Neurointerv Surg. 2019; 11:817–824.
Article27. Hanel RA, Kallmes DF, Lopes DK, Nelson PK, Siddiqui A, Jabbour P, et al. Prospective study on embolization of intracranial aneurysms with the pipeline device: the PREMIER study 1 year results. J Neurointerv Surg. 2020; 12:62–66.
Article28. Bhogal P, Wong K, Uff C, Wadley J, Makalanda HL. The Silk Vista Baby: initial experience and report of two cases. Interv Neuroradiol. 2019; 25:530–538.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Internal Carotid Artery Reconstruction with a “Mega Flow Diverterâ€: First Experience with the 6×50 mm DERIVO Embolization Device
- The Role of Distal Protection Devices for Cardiovascular Intervention
- Inadvertent Complication of a Pipeline Embolization Device for Treatment with Vertebral Artery Dissecting Aneurysm : Distal Tip Fracture of Delivery Wire
- Subacute, Silent Embolization of Amplatzer Atrial Septal Defect Closure Device to the Pulmonary Artery
- Ten Years of Clinical Evaluation of the Woven EndoBridge: A Safe and Effective Treatment for Wide-Neck Bifurcation Aneurysms