Endocrinol Metab.
2021 Oct;36(5):965-973. 10.3803/EnM.2021.1192.
Adrenal Venous Sampling for Subtype Diagnosis of Primary Hyperaldosteronism
- Affiliations
-
- 1Endocrine Center and Clinical Research Center, Ijinkai Takeda General Hospital, Kyoto, Japan
- 2Clinical Research Institute of Endocrinology and Metabolism, NHO Kyoto Medical Center, Tokyo, Japan
- 3Department of Diabetes, Endocrinology and Metabolism, National Center for Global Health and Medicine, Tokyo, Japan
- 4Department of Geriatric and General Medicine, Osaka University Graduate School of Medicine, Osaka, Japan
- 5Department of Health Promotion and Medicine of the Future, Graduate School of Medical Sciences, Kanazawa University, Kanazawa, Japan
- 6Division of Nephrology, Hypertension and Endocrinology, Nihon University School of Medicine, Tokyo, Japan
- 7Department of Diabetes, Endocrinology and Nutrition, Kyoto University Graduate School of Medicine, Kyoto, Japan
- 8Division of Endocrinology and Metabolism, Tottori University Faculty of Medicine, Yonago, Japan
- 9Division of Metabolism and Endocrinology, Department of Internal Medicine, St. Marianna University School of Medicine, Kawasaki, Japan
- KMID: 2521944
- DOI: http://doi.org/10.3803/EnM.2021.1192
Abstract
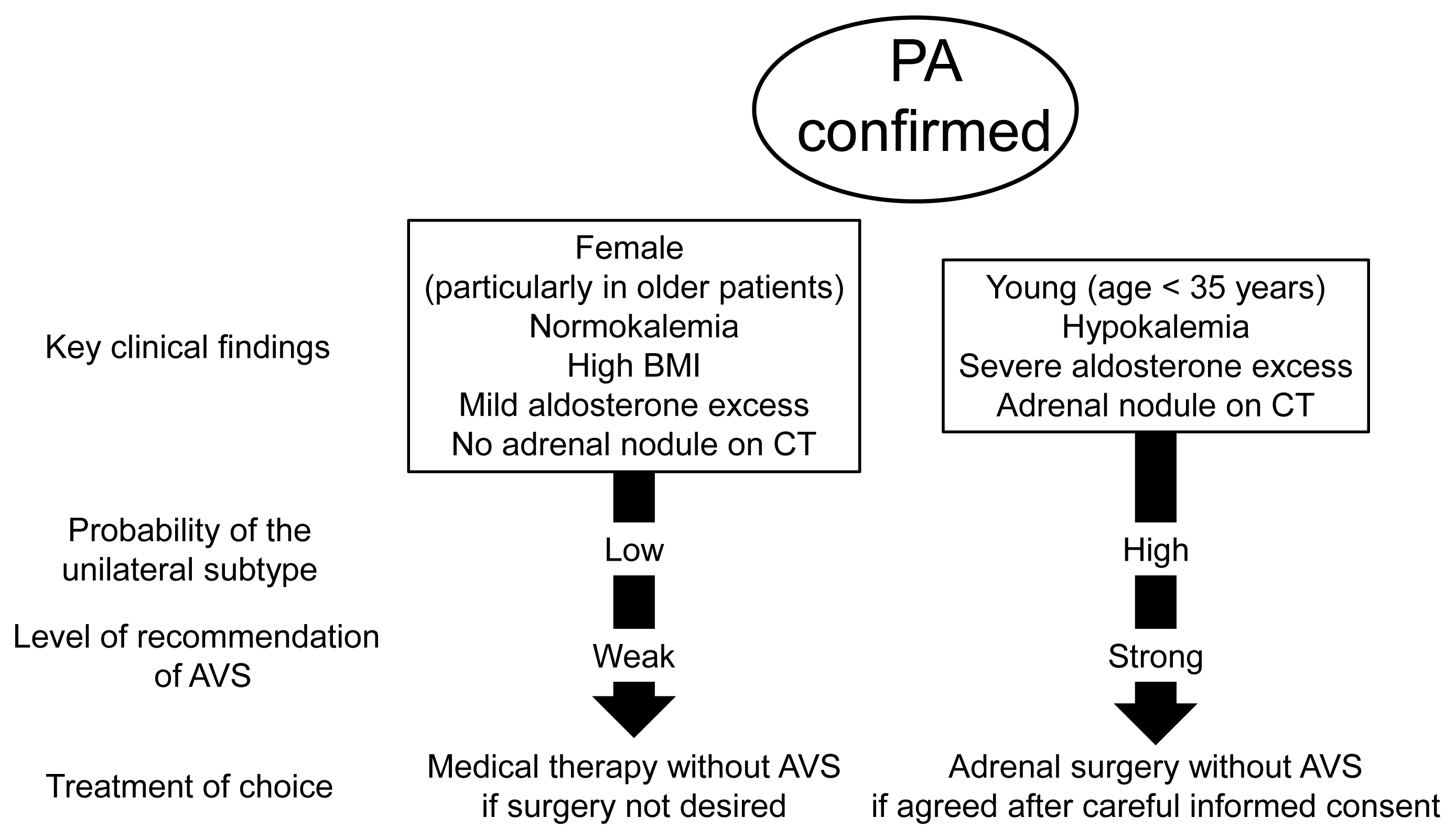
- Adrenal venous sampling (AVS) is the key procedure for lateralization of primary hyperaldosteronism (PA) before surgery. Identification of the adrenal veins using computed tomography (CT) and intraoperative cortisol assay facilitates the success of catheterization. Although administration of adrenocorticotropic hormone (ACTH) has benefits such as improving the success rate, some unilateral cases could be falsely diagnosed as bilateral. Selectivity index of 5 with ACTH stimulation to assess the selectivity of catheterization and lateralization index (LI) >4 with ACTH stimulation for unilateral diagnosis is used in many centers. Co-secretion of cortisol from the tumor potentially affects the lateralization by the LI. Patients aged <35 years with hypokalemia, marked aldosterone excess, and unilateral adrenal nodule on CT have a higher probability of unilateral disease. Patients with normokalemia, mild aldosterone excess, and no adrenal tumor on CT have a higher probability of bilateral disease. Although no methods have 100% specificity for subtype diagnosis that would allow bypassing AVS, prediction of the subtype should be considered when recommending AVS to patients. Methodological standardization and strict indication improve diagnostic quality of AVS. Development of non-invasive imaging and biochemical markers will drive a paradigm shift in the clinical practice of PA.
Figure
Reference
-
1. Conn JW. Presidential address. I. Painting background. II. Primary aldosteronism, a new clinical syndrome. J Lab Clin Med. 1955; 45:3–17.2. Melby JC, Spark RF, Dale SL, Egdahl RH, Kahn PC. Diagnosis and localization of aldosterone-producing adenomas by adrenal-vein cateterization. N Engl J Med. 1967; 277:1050–6.
Article3. Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment. An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016; 101:1889–916.
Article4. Funder JW, Carey RM, Fardella C, Gomez-Sanchez CE, Mantero F, Stowasser M, et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008; 93:3266–81.
Article5. Young WF. Primary aldosteronism: renaissance of a syndrome. Clin Endocrinol (Oxf). 2007; 66:607–18.
Article6. Young WF, Stanson AW. What are the keys to successful adrenal venous sampling (AVS) in patients with primary aldosteronism? Clin Endocrinol (Oxf). 2009; 70:14–7.
Article7. Ota H, Seiji K, Kawabata M, Satani N, Omata K, Ono Y, et al. Dynamic multidetector CT and non-contrast-enhanced MR for right adrenal vein imaging: comparison with catheter venography in adrenal venous sampling. Eur Radiol. 2016; 26:622–30.
Article8. Laurent I, Astere M, Zheng F, Chen X, Yang J, Cheng Q, et al. Adrenal venous sampling with or without adrenocorticotropic hormone stimulation: a meta-analysis. J Clin Endocrinol Metab. 2019; 104:1060–8.
Article9. Rossi GP, Auchus RJ, Brown M, Lenders JW, Naruse M, Plouin PF, et al. An expert consensus statement on use of adrenal vein sampling for the subtyping of primary aldosteronism. Hypertension. 2014; 63:151–60.
Article10. Chang CC, Lee BC, Chang YC, Wu VC, Huang KH, Liu KL, et al. Comparison of C-arm computed tomography and on-site quick cortisol assay for adrenal venous sampling: a retrospective study of 178 patients. Eur Radiol. 2017; 27:5006–14.
Article11. Park CH, Hong N, Han K, Kang SW, Lee CR, Park S, et al. C-Arm computed tomography-assisted adrenal venous sampling improved right adrenal vein cannulation and sampling quality in primary aldosteronism. Endocrinol Metab (Seoul). 2018; 33:236–44.
Article12. Stowasser M. Improving the success and reliability of adrenal venous sampling: focus on intraprocedural cortisol measurement. Clin Chem. 2012; 58:1275–7.
Article13. Yoneda T, Karashima S, Kometani M, Usukura M, Demura M, Sanada J, et al. Impact of new quick gold nanoparticle-based cortisol assay during adrenal vein sampling for primary aldosteronism. J Clin Endocrinol Metab. 2016; 101:2554–61.
Article14. Seccia TM, Miotto D, Battistel M, Motta R, Barisa M, Maniero C, et al. A stress reaction affects assessment of selectivity of adrenal venous sampling and of lateralization of aldosterone excess in primary aldosteronism. Eur J Endocrinol. 2012; 166:869–75.
Article15. Rossi GP, Barisa M, Allolio B, Auchus RJ, Amar L, Cohen D, et al. The Adrenal Vein Sampling International Study (AVIS) for identifying the major subtypes of primary aldosteronism. J Clin Endocrinol Metab. 2012; 97:1606–14.
Article16. Rossi GP, Pitter G, Bernante P, Motta R, Feltrin G, Miotto D. Adrenal vein sampling for primary aldosteronism: the assessment of selectivity and lateralization of aldosterone excess baseline and after adrenocorticotropic hormone (ACTH) stimulation. J Hypertens. 2008; 26:989–97.
Article17. Takeda Y, Umakoshi H, Takeda Y, Yoneda T, Kurihara I, Katabami T, et al. Impact of adrenocorticotropic hormone stimulation during adrenal venous sampling on outcomes of primary aldosteronism. J Hypertens. 2019; 37:1077–82.
Article18. Deinum J, Groenewoud H, van der Wilt GJ, Lenzini L, Rossi GP. Adrenal venous sampling: cosyntropin stimulation or not? Eur J Endocrinol. 2019; 181:D15–26.
Article19. Satoh F, Abe T, Tanemoto M, Nakamura M, Abe M, Uruno A, et al. Localization of aldosterone-producing adrenocortical adenomas: significance of adrenal venous sampling. Hypertens Res. 2007; 30:1083–95.
Article20. Monticone S, Satoh F, Giacchetti G, Viola A, Morimoto R, Kudo M, et al. Effect of adrenocorticotropic hormone stimulation during adrenal vein sampling in primary aldosteronism. Hypertension. 2012; 59:840–6.
Article21. Kobayashi H, Nakamura Y, Abe M, Kurihara I, Itoh H, Ichijo T, et al. Effect of cosyntropin during adrenal venous sampling on subtype of primary aldosteronism: analysis of surgical outcome. Eur J Endocrinol. 2020; 182:265–73.
Article22. Rossitto G, Maiolino G, Lenzini L, Bisogni V, Seccia TM, Cesari M, et al. Subtyping of primary aldosteronism with adrenal vein sampling: hormone- and side-specific effects of cosyntropin and metoclopramide. Surgery. 2018; 163:789–95.
Article23. Rossitto G, Amar L, Azizi M, Riester A, Reincke M, Degenhart C, et al. Subtyping of primary aldosteronism in the AVIS-2 Study: assessment of selectivity and lateralization. J Clin Endocrinol Metab. 2020; 105:dgz017.
Article24. Rossi GP, Sacchetto A, Chiesura-Corona M, De Toni R, Gallina M, Feltrin GP, et al. Identification of the etiology of primary aldosteronism with adrenal vein sampling in patients with equivocal computed tomography and magnetic resonance findings: results in 104 consecutive cases. J Clin Endocrinol Metab. 2001; 86:1083–90.
Article25. Ceral J, Solar M, Krajina A, Ballon M, Suba P, Cap J. Adrenal venous sampling in primary aldosteronism: a low dilution of adrenal venous blood is crucial for a correct interpretation of the results. Eur J Endocrinol. 2010; 162:101–7.
Article26. Akehi Y, Yanase T, Motonaga R, Umakoshi H, Tsuiki M, Takeda Y, et al. High prevalence of diabetes in patients with primary aldosteronism (PA) associated with subclinical hypercortisolism and prediabetes more prevalent in bilateral than unilateral PA: a large, multicenter cohort study in Japan. Diabetes Care. 2019; 42:938–45.
Article27. Gerards J, Heinrich DA, Adolf C, Meisinger C, Rathmann W, Sturm L, et al. Impaired glucose metabolism in primary aldosteronism is associated with cortisol cosecretion. J Clin Endocrinol Metab. 2019; 104:3192–202.
Article28. Lenders JWM, Eisenhofer G, Reincke M. Subtyping of patients with primary aldosteronism: an update. Horm Metab Res. 2017; 49:922–8.
Article29. Goupil R, Wolley M, Ahmed AH, Gordon RD, Stowasser M. Does concomitant autonomous adrenal cortisol overproduction have the potential to confound the interpretation of adrenal venous sampling in primary aldosteronism? Clin Endocrinol (Oxf). 2015; 83:456–61.
Article30. O’Toole SM, Sze WC, Chung TT, Akker SA, Druce MR, Waterhouse M, et al. Low-grade cortisol cosecretion has limited impact on ACTH-stimulated AVS parameters in primary aldosteronism. J Clin Endocrinol Metab. 2020; 105:dgaa519.
Article31. Williams TA, Lenders JWM, Mulatero P, Burrello J, Rottenkolber M, Adolf C, et al. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. 2017; 5:689–99.
Article32. Takeda M, Yamamoto K, Akasaka H, Rakugi H, Naruse M, Takeda Y, et al. Clinical characteristics and postoperative outcomes of primary aldosteronism in the elderly. J Clin Endocrinol Metab. 2018; 103:3620–9.
Article33. Nishikawa T, Omura M, Satoh F, Shibata H, Takahashi K, Tamura N, et al. Guidelines for the diagnosis and treatment of primary aldosteronism: the Japan Endocrine Society 2009. Endocr J. 2011; 58:711–21.
Article34. Umakoshi H, Naruse M, Wada N, Ichijo T, Kamemura K, Matsuda Y, et al. Adrenal venous sampling in patients with positive screening but negative confirmatory testing for primary aldosteronism. Hypertension. 2016; 67:1014–9.
Article35. Kline GA, Chin A, So B, Harvey A, Pasieka JL. Defining contralateral adrenal suppression in primary aldosteronism: implications for diagnosis and outcome. Clin Endocrinol (Oxf). 2015; 83:20–7.
Article36. Wolley MJ, Gordon RD, Ahmed AH, Stowasser M. Does contralateral suppression at adrenal venous sampling predict outcome following unilateral adrenalectomy for primary aldosteronism? A retrospective study. J Clin Endocrinol Metab. 2015; 100:1477–84.
Article37. Umakoshi H, Tanase-Nakao K, Wada N, Ichijo T, Sone M, Inagaki N, et al. Importance of contralateral aldosterone suppression during adrenal vein sampling in the subtype evaluation of primary aldosteronism. Clin Endocrinol (Oxf). 2015; 83:462–7.
Article38. Lee J, Kang B, Ha J, Kim MH, Choi B, Hong TH, et al. Clinical outcomes of primary aldosteronism based on lateralization index and contralateral suppression index after adrenal venous sampling in real-world practice: a retrospective cohort study. BMC Endocr Disord. 2020; 20:114.
Article39. Lee SE, Kim JH, Lee YB, Seok H, Shin IS, Eun YH, et al. Bilateral adrenocortical masses producing aldosterone and cortisol independently. Endocrinol Metab (Seoul). 2015; 30:607–13.
Article40. Pasternak JD, Epelboym I, Seiser N, Wingo M, Herman M, Cowan V, et al. Diagnostic utility of data from adrenal venous sampling for primary aldosteronism despite failed cannulation of the right adrenal vein. Surgery. 2016; 159:267–73.
Article41. Wang TS, Kline G, Yen TW, Yin Z, Liu Y, Rilling W, et al. A multi-institutional comparison of adrenal venous sampling in patients with primary aldosteronism: caution advised if successful bilateral adrenal vein sampling is not achieved. World J Surg. 2018; 42:466–72.
Article42. Strajina V, Al-Hilli Z, Andrews JC, Bancos I, Thompson GB, Farley DR, et al. Primary aldosteronism: making sense of partial data sets from failed adrenal venous sampling-suppression of adrenal aldosterone production can be used in clinical decision making. Surgery. 2018; 163:801–6.
Article43. Fujii Y, Umakoshi H, Wada N, Ichijo T, Kamemura K, Matsuda Y, et al. Subtype prediction of primary aldosteronism by combining aldosterone concentrations in the left adrenal vein and inferior vena cava: a multicenter collaborative study on adrenal venous sampling. J Hum Hypertens. 2017; 32:12–9.
Article44. Umakoshi H, Ogasawara T, Takeda Y, Kurihara I, Itoh H, Katabami T, et al. Accuracy of adrenal computed tomography in predicting the unilateral subtype in young patients with hypokalaemia and elevation of aldosterone in primary aldosteronism. Clin Endocrinol (Oxf). 2018; 88:645–51.
Article45. Riester A, Fischer E, Degenhart C, Reiser MF, Bidlingmaier M, Beuschlein F, et al. Age below 40 or a recently proposed clinical prediction score cannot bypass adrenal venous sampling in primary aldosteronism. J Clin Endocrinol Metab. 2014; 99:E1035–9.
Article46. Umakoshi H, Tsuiki M, Takeda Y, Kurihara I, Itoh H, Katabami T, et al. Significance of computed tomography and serum potassium in predicting subtype diagnosis of primary aldosteronism. J Clin Endocrinol Metab. 2018; 103:900–8.
Article47. Ohno Y, Sone M, Inagaki N, Yamasaki T, Ogawa O, Takeda Y, et al. Obesity as a key factor underlying idiopathic hyperaldosteronism. J Clin Endocrinol Metab. 2018; 103:4456–64.
Article48. Akasaka H, Yamamoto K, Rakugi H, Nagasawa M, Nakamaru R, Ichijo T, et al. Sex difference in the association between subtype distribution and age at diagnosis in patients with primary aldosteronism. Hypertension. 2019; 74:368–74.
Article49. Kobayashi H, Abe M, Soma M, Takeda Y, Kurihara I, Itoh H, et al. Development and validation of subtype prediction scores for the workup of primary aldosteronism. J Hypertens. 2018; 36:2269–76.
Article50. Xiao L, Jiang Y, Zhang C, Jiang L, Zhou W, Su T, et al. A novel clinical nomogram to predict bilateral hyperaldosteronism in Chinese patients with primary aldosteronism. Clin Endocrinol (Oxf). 2019; 90:781–8.
Article51. Nomura K, Kusakabe K, Maki M, Ito Y, Aiba M, Demura H. Iodomethylnorcholesterol uptake in an aldosteronoma shown by dexamethasone-suppression scintigraphy: relationship to adenoma size and functional activity. J Clin Endocrinol Metab. 1990; 71:825–30.
Article52. Yen RF, Wu VC, Liu KL, Cheng MF, Wu YW, Chueh SC, et al. 131I-6beta-iodomethyl-19-norcholesterol SPECT/CT for primary aldosteronism patients with inconclusive adrenal venous sampling and CT results. J Nucl Med. 2009; 50:1631–7.
Article53. Burton TJ, Mackenzie IS, Balan K, Koo B, Bird N, Soloviev DV, et al. Evaluation of the sensitivity and specificity of (11)C-metomidate positron emission tomography (PET)-CT for lateralizing aldosterone secretion by Conn’s adenomas. J Clin Endocrinol Metab. 2012; 97:100–9.54. Soinio M, Luukkonen AK, Seppanen M, Kemppainen J, Seppanen J, Pienimaki JP, et al. Functional imaging with 11C-metomidate PET for subtype diagnosis in primary aldosteronism. Eur J Endocrinol. 2020; 183:539–50.
Article55. Ding J, Zhang Y, Wen J, Zhang H, Wang H, Luo Y, et al. Imaging CXCR4 expression in patients with suspected primary hyperaldosteronism. Eur J Nucl Med Mol Imaging. 2020; 47:2656–65.
Article56. Abe T, Naruse M, Young WF Jr, Kobashi N, Doi Y, Izawa A, et al. A novel CYP11B2-specific imaging agent for detection of unilateral subtypes of primary aldosteronism. J Clin Endocrinol Metab. 2016; 101:1008–15.
Article57. Sander K, Gendron T, Cybulska KA, Sirindil F, Zhou J, Kalber TL, et al. Development of [18F]AldoView as the first highly selective aldosterone synthase PET tracer for imaging of primary hyperaldosteronism. J Med Chem. 2021; 64:9321–29.58. Eisenhofer G, Duran C, Cannistraci CV, Peitzsch M, Williams TA, Riester A, et al. Use of steroid profiling combined with machine learning for identification and subtype classification in primary aldosteronism. JAMA Netw Open. 2020; 3:e2016209.
Article59. Satoh F, Morimoto R, Ono Y, Iwakura Y, Omata K, Kudo M, et al. Measurement of peripheral plasma 18-oxocortisol can discriminate unilateral adenoma from bilateral diseases in patients with primary aldosteronism. Hypertension. 2015; 65:1096–102.
Article60. Tezuka Y, Yamazaki Y, Kitada M, Morimoto R, Kudo M, Seiji K, et al. 18-Oxocortisol synthesis in aldosterone-producing adrenocortical adenoma and significance of KCNJ5 mutation status. Hypertension. 2019; 73:1283–90.
Article61. Nakano Y, Yoshimoto T, Watanabe R, Murakami M, Fukuda T, Saito K, et al. miRNA299 involvement in CYP11B2 expression in aldosterone-producing adenoma. Eur J Endocrinol. 2019; 181:69–78.
Article62. Tombol Z, Turai PI, Decmann A, Igaz P. MicroRNAs and adrenocortical tumors: where do we stand on primary aldosteronism? Horm Metab Res. 2020; 52:394–403.
Article63. Ohno Y, Naruse M, Beuschlein F, Schreiner F, Parasiliti-Caprino M, Deinum J, et al. Adrenal venous sampling-guided adrenalectomy rates in primary aldosteronism: results of an international cohort (AVSTAT). J Clin Endocrinol Metab. 2021; 106:e1400–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A case of idiopathic hyperaldosteronism vs. bilateral aldosterone producing adenoma
- Primary Aldosteronism Due to Aldosterone Producing Adenama in the Presence of Contralateral Nonfunctioning Adenama
- Anatomical Variations Encountered during Adrenal Venous Sampling: A Report of Three Case Series and Review of Literature
- Clinical Experiences of Adrenal Tumors: Studies on the Localization of Adrenal Tumors
- C-Arm Computed Tomography-Assisted Adrenal Venous Sampling Improved Right Adrenal Vein Cannulation and Sampling Quality in Primary Aldosteronism