Intest Res.
2021 Oct;19(4):448-460. 10.5217/ir.2020.00026.
Efficacy and safety of a new vedolizumab subcutaneous formulation in Japanese patients with moderately to severely active ulcerative colitis
- Affiliations
-
- 1Center for Advanced IBD Research and Treatment, Kitasato University Kitasato Institute Hospital, Tokyo, Japan
- 2Infusion clinic, Osaka, Japan
- 3Inflammatory Bowel Disease Center, Sapporo Tokushukai Hospital, Sapporo, Japan
- 4Yokoyama IBD Clinic, Nagoya, Japan
- 5Department of Gastroenterology and Hepatology, Tokyo Dental Medical University Hospital, Tokyo, Japan
- 6Department of Gastroenterology, Kagawa Prefectural Central Hospital, Kagawa, Japan
- 7Takeda Development Center Japan, Takeda Pharmaceutical Company Limited, Osaka, Japan
- 8Japan Medical Office, Takeda Pharmaceutical Company Limited, Tokyo, Japan
- KMID: 2521608
- DOI: http://doi.org/10.5217/ir.2020.00026
Abstract
- Background/Aims
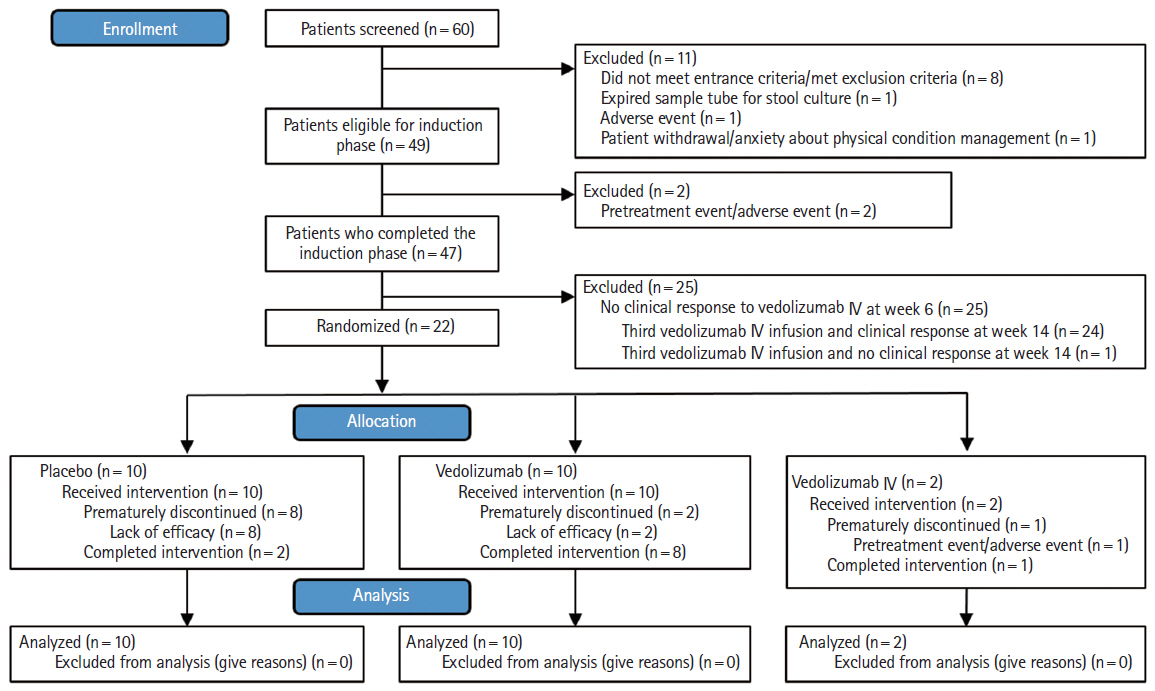
A subgroup analysis was conducted in Japanese patients with moderate to severe ulcerative colitis (UC) enrolled in the phase 3 VISIBLE 1 study, which evaluated the safety and efficacy of a new vedolizumab subcutaneous (SC) formulation.
Methods
Eligible patients received open-label infusions of vedolizumab 300 mg intravenous (IV) at weeks 0 and 2 in the induction phase. Patients with clinical response by complete Mayo score at week 6 entered the double-blind maintenance phase and were randomized to vedolizumab 108 mg SC every 2 weeks, placebo, or vedolizumab 300 mg IV every 8 weeks. The primary endpoint was clinical remission (complete Mayo score ≤ 2 points; no individual subscore > 1 point) at week 52.
Results
Of 49 patients who entered the induction phase, 22 out of 49 patients (45%) had clinical response at week 6 and were randomized to vedolizumab 108 mg SC (n = 10), placebo (n = 10), or vedolizumab 300 mg IV (n = 2). At week 52, 4 out of 10 patients (40%) who received vedolizumab SC had clinical remission versus 2 out of 10 patients (20%) who received placebo (difference: 20% [95% confidence interval, –27.9 to 61.8]). Two patients (2/10, 20%) who received vedolizumab SC experienced an injection-site reaction versus none who received placebo.
Conclusions
Our results indicate that the efficacy of vedolizumab SC in a subgroup of Japanese patients with UC are similar with those in the overall VISIBLE 1 study population, and with those established with vedolizumab IV. The safety and tolerability of vedolizumab SC were generally similar to that established for vedolizumab IV. (ClinicalTrials.gov ID NCT02611830; EudraCT 2015-000480-14)
Figure
Reference
-
1. Hibi T, Imai Y, Senoo A, Ohta K, Ukyo Y. Efficacy and safety of golimumab 52-week maintenance therapy in Japanese patients with moderate to severely active ulcerative colitis: a phase 3, double-blind, randomized, placebo-controlled study (PURSUIT-J study). J Gastroenterol. 2017; 52:1101–1111.2. Testa A, Castiglione F, Nardone OM, Colombo GL. Adherence in ulcerative colitis: an overview. Patient Prefer Adherence. 2017; 11:297–303.
Article3. Bittner B, Richter W, Schmidt J. Subcutaneous administration of biotherapeutics: an overview of current challenges and opportunities. BioDrugs. 2018; 32:425–440.
Article4. Pharmaceuticals and Medical Devices Agency. Vedolizumab prescribing information [Internet]. c2019 [cited 2020 May 26]. https://www.info.pmda.go.jp/go/pack/2399405F1020_1_03/.5. Pharmaceuticals and Medical Devices Agency. Infliximab prescribing information [Internet]. c2019 [cited 2020 May 26]. https://www.info.pmda.go.jp/go/pack/2399402F1026_1_43/.6. Pharmaceuticals and Medical Devices Agency. Adalimumab prescribing information [Internet]. c2020 [cited 2020 May 26]. https://www.info.pmda.go.jp/go/pack/3999459G1029_1_02/.7. Pharmaceuticals and Medical Devices Agency. Golimumab prescribing information [Internet]. c2020 [cited 2020 May 26]. https://www.info.pmda.go.jp/go/pack/3999433G1024_1_21/.8. Pharmaceuticals and Medical Devices Agency. Ustekinumab prescribing information [Internet]. c2020 [cited 2020 May 26]. https://www.info.pmda.go.jp/go/pack/3999431G1025_1_17/.9. Soler D, Chapman T, Yang LL, Wyant T, Egan R, Fedyk ER. The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther. 2009; 330:864–875.10. Wyant T, Fedyk E, Abhyankar B. An overview of the mechanism of action of the monoclonal antibody vedolizumab. J Crohns Colitis. 2016; 10:1437–1444.11. Motoya S, Watanabe K, Ogata H, et al. Vedolizumab in Japanese patients with ulcerative colitis: a phase 3, randomized, double-blind, placebo-controlled study. PLoS One. 2019; 14:e0212989.12. Watanabe K, Motoya S, Ogata H, et al. Effects of vedolizumab in Japanese patients with Crohn’s disease: a prospective, multicenter, randomized, placebo-controlled phase 3 trial with exploratory analyses. J Gastroenterol. 2020; 55:291–306.13. Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013; 369:699–710.14. Kobayashi T, Uda A, Udagawa E, Hibi T. Lack of increased risk of lymphoma by thiopurines or biologics in Japanese patients with inflammatory bowel disease: a large-scale administrative database analysis. J Crohns Colitis. 2020; 14:617–623.15. Beaugerie L, Brousse N, Bouvier AM, et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet. 2009; 374:1617–1625.16. Pasternak B, Svanström H, Schmiegelow K, Jess T, Hviid A. Use of azathioprine and the risk of cancer in inflammatory bowel disease. Am J Epidemiol. 2013; 177:1296–1305.17. Sandborn WJ, Baert F, Danese S, et al. Efficacy and safety of vedolizumab subcutaneous formulation in a randomized trial of patients with ulcerative colitis. Gastroenterology. 2020; 158:562–572.18. Rosario M, Yang LL, Wyant T. P010 Results from a new antivedolizumab antibody assay. Am J Gastroenterol. 2019; 114:S3.19. Hibi T, Imai Y, Murata Y, Matsushima N, Zheng R, Gasink C. Efficacy and safety of ustekinumab in Japanese patients with moderately to severely active Crohn’s disease: a subpopulation analysis of phase 3 induction and maintenance studies. Intest Res. 2017; 15:475–486.20. AbbVie Inc. HUMIRA® (Adalimumab) prescribing information. North Chicago: AbbVie Inc.;2018.21. UCB Inc. CIMZIA® (certolizumab pegol) prescribing information. Smyrna: UCB Inc.;2018.22. Janssen Biotech Inc. SIMPONI® (golimumab) prescribing information. Horsham: Janssen Biotech Inc.;2018.23. Colombel JF, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut. 2017; 66:839–851.
Article24. Vermeire S, Gils A, Accossato P, Lula S, Marren A. Immunogenicity of biologics in inflammatory bowel disease. Therap Adv Gastroenterol. 2018; 11:1756283–X17750355.
Article25. Feagan BG, Patel H, Colombel JF, et al. Effects of vedolizumab on health-related quality of life in patients with ulcerative colitis: results from the randomised GEMINI 1 trial. Aliment Pharmacol Ther. 2017; 45:264–275.
Article26. Hibi T, Motoya S, Ashida T, et al. Efficacy and safety of abrilumab, an α4β7 integrin inhibitor, in Japanese patients with moderate-to-severe ulcerative colitis: a phase II study. Intest Res. 2019; 17:375–386.
Article27. Suzuki Y, Motoya S, Hanai H, et al. Efficacy and safety of adalimumab in Japanese patients with moderately to severely active ulcerative colitis. J Gastroenterol. 2014; 49:283–294.28. Sands BE, Peyrin-Biroulet L, Loftus EV, et al. 416a –Vedolizumab shows superior efficacy versus adalimumab: results of varsity—the first head-to-head study of biologic therapy for moderate-to-severe ulcerative colitis. Gastroenterol. 2019; 156:S–81.29. Morishige R, Nakajima H, Yoshizawa K, Mahlich J, Sruamsiri R. Preferences regarding shared decision-making in Japanese inflammatory bowel disease patients. Adv Ther. 2017; 33:2242–2256.
Article30. Mahlich J, Matsuoka K, Sruamsiri R. Shared decision making and treatment satisfaction in Japanese patients with inflammatory bowel disease. Dig Dis. 2017; 35:454–462.
Article31. Hirai F, Watanabe K, Matsumoto T, et al. Patients’ assessment of adalimumab self-injection for Crohn’s disease: a multicenter questionnaire survey (The PEARL Survey). Hepatogastroenterology. 2014; 61:1654–1660.32. Mahlich J, Sruamsiri R. Treatment patterns of rheumatoid arthritis in Japanese hospitals and predictors of the initiation of biologic agents. Curr Med Res Opin. 2017; 33:101–107.33. Striesow F, Brandt A. Preference, satisfaction and usability of subcutaneously administered methotrexate for rheumatoid arthritis or psoriatic arthritis: results of a postmarketing surveillance study with a high-concentration formulation. Ther Adv Musculoskelet Dis. 2012; 4:3–9.
Article34. Kobayashi T, Udagawa E, Uda A, Hibi T, Hisamatsu T. Impact of immunomodulator use on treatment persistence in patients with ulcerative colitis: a claims database analysis. J Gastroenterol Hepatol. 2020; 35:225–232.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy and safety of mirikizumab as induction and maintenance therapy for Japanese patients with moderately to severely active ulcerative colitis: a subgroup analysis of the global phase 3 LUCENT-1 and LUCENT-2 studies
- Efficacy of biologic therapies for biologic-naïve Japanese patients with moderately to severely active ulcerative colitis: a network meta-analysis
- Efficacy and safety of vedolizumab in ulcerative colitis in patients from Asian countries in the GEMINI 1 study
- Population pharmacokinetics of vedolizumab in Asian and non-Asian patients with ulcerative colitis and Crohn’s disease
- Emerging Therapies: What Are Promising in the Near Future?