Intest Res.
2021 Jan;19(1):95-105. 10.5217/ir.2019.09167.
Population pharmacokinetics of vedolizumab in Asian and non-Asian patients with ulcerative colitis and Crohn’s disease
- Affiliations
-
- 1Takeda PRA Development Center KK, Osaka, Japan
- 2Metrum Research Group, Tariffville, CT, USA
- 3Takeda Development Center Americas Inc, Cambridge, MA, USA
- 4Takeda Pharmaceutical Company Limited, Osaka, Japan
- 5Center for Advanced IBD Research and Treatment, Kitasato University Kitasato Institute Hospital, Tokyo, Japan
- KMID: 2512105
- DOI: http://doi.org/10.5217/ir.2019.09167
Abstract
- Background/Aims
Vedolizumab is indicated for moderately-to-severely active ulcerative colitis (UC) and Crohn’s disease (CD). Because multiple factors may result in different pharmacokinetics and clinical efficacies, understanding determinants of vedolizumab clearance may enhance dose and treatment strategies. The aim was to characterize vedolizumab pharmacokinetics in Asian and non-Asian UC and CD patients.
Methods
Population pharmacokinetic analysis for repeated measures, using data from 5 studies, was conducted using nonlinear mixed-effects modeling. A Bayesian estimation approach in NONMEM 7.3 was utilized to leverage the predominantly sparse data available for this analysis with results from a prior population pharmacokinetic analysis of vedolizumab.
Results
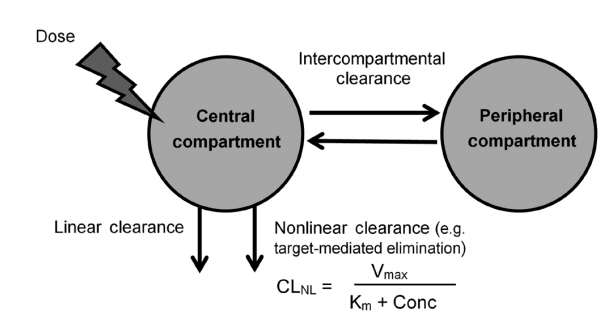
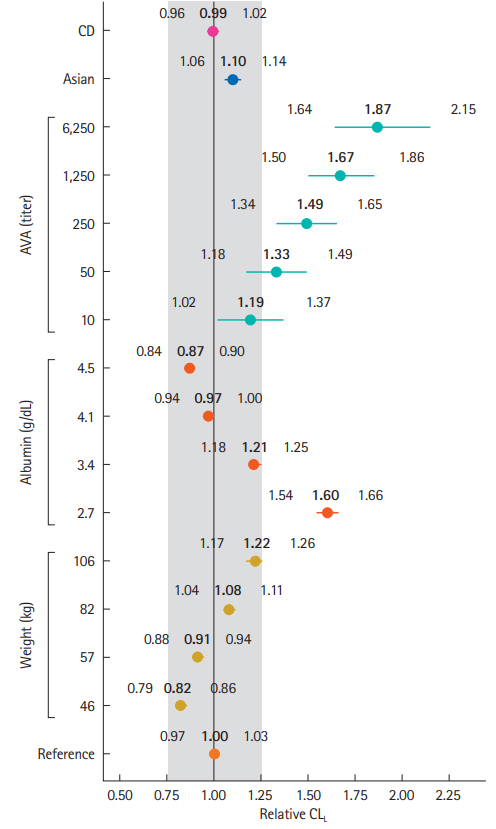
Vedolizumab pharmacokinetics were described by a 2-compartment model with parallel linear and nonlinear elimination. Using reference covariate values, linear elimination half life of vedolizumab was 24.7 days for anti-vedolizumab antibody (AVA)-negative patients and 18.1 days for AVA-positive patients; linear clearance (CLL) was 0.165 L/day for AVA-negative patients and 0.246 L/day for AVA-positive patients; central (Vc) and peripheral compartment volumes of distribution were 3.16 L and 1.84 L, respectively. Interindividual variabilities (percent coefficient of variation) were 30.8% for CLL and 19% for Vc; interoccasion variability on CLL was 20.3%; residual variance was 17.8%. For albumin, body weight and AVA, only extreme values were identified as potentially clinically important predictors of CLL. The effect of race (Asian/non-Asian) and diagnosis (UC/CD) on CLL was negligible and likely not of clinical importance.
Conclusions
Pharmacokinetic parameters were similar in Asian and non-Asian patients with moderately-to-severely active UC and CD. This analysis supports use of vedolizumab flat-fixed dosing in these patients. (Clinicaltrials.gov Identifiers: NCT00783718 (GEMINI 1); NCT00783692 (GEMINI 2). CCT 101; NCT02039505 and CCT-001; NCT02038920)
Figure
Cited by 2 articles
-
Vedolizumab does not increase perioperative surgical complications in patients with inflammatory bowel disease, cohort study
Vitaliy Y. Poylin, Jose Cataneo Serrato, Jonathan Pastrana Del Valle, Joseph D. Feuerstein
Intest Res. 2022;20(1):72-77. doi: 10.5217/ir.2020.00117.Natural history of inflammatory bowel disease: a comparison between the East and the West
Eun Mi Song, Suk-Kyun Yang
Intest Res. 2022;20(4):418-430. doi: 10.5217/ir.2021.00104.
Reference
-
1. Soler D, Chapman T, Yang LL, Wyant T, Egan R, Fedyk ER. The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther. 2009; 330:864–875.
Article2. U.S. Food and Drug Administration. Takeda Pharmaceuticals America Inc. Entyvio (vedolizumab) prescribing information [Internet]. c2014 [cited 2019 May 10]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/125476s000lbl.pdf.3. European Medicine Agency. Takeda Pharma A/S. Entyvio (vedolizumab) summary of product characteristics [Internet]. c2018 [cited 2019 May 10]. https://www.ema.europa.eu/en/documents/product-information/entyvio-epar-product-information_en.pdf. 2019.4. Rosario M, Dirks NL, Gastonguay MR, et al. Population pharmacokinetics-pharmacodynamics of vedolizumab in patients with ulcerative colitis and Crohn’s disease. Aliment Pharmacol Ther. 2015; 42:188–202.
Article5. Zhu Y, Hu C, Lu M, et al. Population pharmacokinetic modeling of ustekinumab, a human monoclonal antibody targeting IL-12/23p40, in patients with moderate to severe plaque psoriasis. J Clin Pharmacol. 2009; 49:162–175.
Article6. Fasanmade AA, Adedokun OJ, Ford J, et al. Population pharmacokinetic analysis of infliximab in patients with ulcerative colitis. Eur J Clin Pharmacol. 2009; 65:1211–1228.
Article7. Gibiansky L, Gibiansky E. Target-mediated drug disposition model: relationships with indirect response models and application to population PK-PD analysis. J Pharmacokinet Pharmacodyn. 2009; 36:341–351.
Article8. Dirks NL, Meibohm B. Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010; 49:633–659.
Article9. Ordás I, Feagan BG, Sandborn WJ. Therapeutic drug monitoring of tumor necrosis factor antagonists in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2012; 10:1079–1087.
Article10. Fasanmade AA, Adedokun OJ, Olson A, Strauss R, Davis HM. Serum albumin concentration: a predictive factor of infliximab pharmacokinetics and clinical response in patients with ulcerative colitis. Int J Clin Pharmacol Ther. 2010; 48:297–308.
Article11. Matsushima S, Huang Y, Suzuki H, Nishino J, Lloyd P. Ethnic sensitivity assessment: pharmacokinetic comparability between Japanese and non-Japanese healthy subjects on selected mAbs. Expert Opin Drug Metab Toxicol. 2015; 11:179–191.
Article12. Chiba K, Yoshitsugu H, Kyosaka Y, et al. A comprehensive review of the pharmacokinetics of approved therapeutic monoclonal antibodies in Japan: are Japanese phase I studies still needed? J Clin Pharmacol. 2014; 54:483–494.
Article13. Ling J, Lyn S, Xu Z, et al. Lack of racial differences in the pharmacokinetics of subcutaneous golimumab in healthy Japanese and Caucasian male subjects. J Clin Pharmacol. 2010; 50:792–802.
Article14. Bajaj G, Wang X, Agrawal S, Gupta M, Roy A, Feng Y. Modelbased population pharmacokinetic analysis of nivolumab in patients with solid tumors. CPT Pharmacometrics Syst Pharmacol. 2017; 6:58–66.
Article15. Yee KL, Kleijn HJ, Kerbusch T, et al. Population pharmacokinetics and pharmacodynamics of bezlotoxumab in adults with primary and recurrent Clostridium difficile infection. Antimicrob Agents Chemother. 2019; 63:e01971. –18.
Article16. Wade JR, Parker G, Kosutic G, et al. Population pharmacokinetic analysis of certolizumab pegol in patients with Crohn’s disease. J Clin Pharmacol. 2015; 55:866–874.
Article17. Parikh A, Leach T, Wyant T, et al. Vedolizumab for the treatment of active ulcerative colitis: a randomized controlled phase 2 dose-ranging study. Inflamm Bowel Dis. 2012; 18:1470–1479.
Article18. Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013; 369:699–710.
Article19. Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013; 369:711–721.
Article20. Kobayashi K, Suzuki Y, Watanabe K, et al. A phase 1, multipledose study of vedolizumab in Japanese patients with ulcerative colitis. J Clin Pharmacol. 2019; 59:271–279.
Article21. Motoya S, Watanabe K, Ogata H, et al. Vedolizumab in Japanese patients with ulcerative colitis: a phase 3, randomized, doubleblind, placebo-controlled study. PLoS One. 2019; 14:e0212989.
Article22. Beal SL, Sheiner LB, BoecKmann AJ. NONMEM Users GuidesPart I-VIII. 6th ed. Ellicott City;ICON Development Solutions: 2012.23. Rosario M, Yang L, Wyant T. P010 Results from a new anti-vedolizumab antibody assay. Am J Gastroenterol. 2019; 114:S3.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Efficacy of Vedolizumab, an alpha4beta7 Integrin Antibody, in Crohn's Disease and Ulcerative Colitis
- Efficacy and safety of vedolizumab in ulcerative colitis in patients from Asian countries in the GEMINI 1 study
- Combined eosinophilic gastroenteritis and ulcerative colitis successfully treated by vedolizumab: a case report
- Pharmacologic treatment for inflammatory bowel disease
- Treatment of inflammatory bowel diseases: focusing on biologic agents and new therapies