Korean J Physiol Pharmacol.
2021 Sep;25(5):449-457. 10.4196/kjpp.2021.25.5.449.
Melatonin modulates nitric oxide-regulated WNK-SPAK/OSR1-NKCC1 signaling in dorsal raphe nucleus of rats
- Affiliations
-
- 1Department of Biomedical Science, Graduate School, Kyung Hee University, Seoul 02447, Korea.
- 2Department of Physiology, College of Medicine, Kyung Hee University, Seoul 02447, Korea.
- 3Biomedical Science Institute and Medical Research Center for Reactive Oxygen Species, College of Medicine, Kyung Hee University, Seoul 02447, Korea.
- 4Department of Biochemistry and Molecular Biology, College of Medicine, Kyung Hee University, Seoul 02447, Korea.
- KMID: 2519407
- DOI: http://doi.org/10.4196/kjpp.2021.25.5.449
Abstract
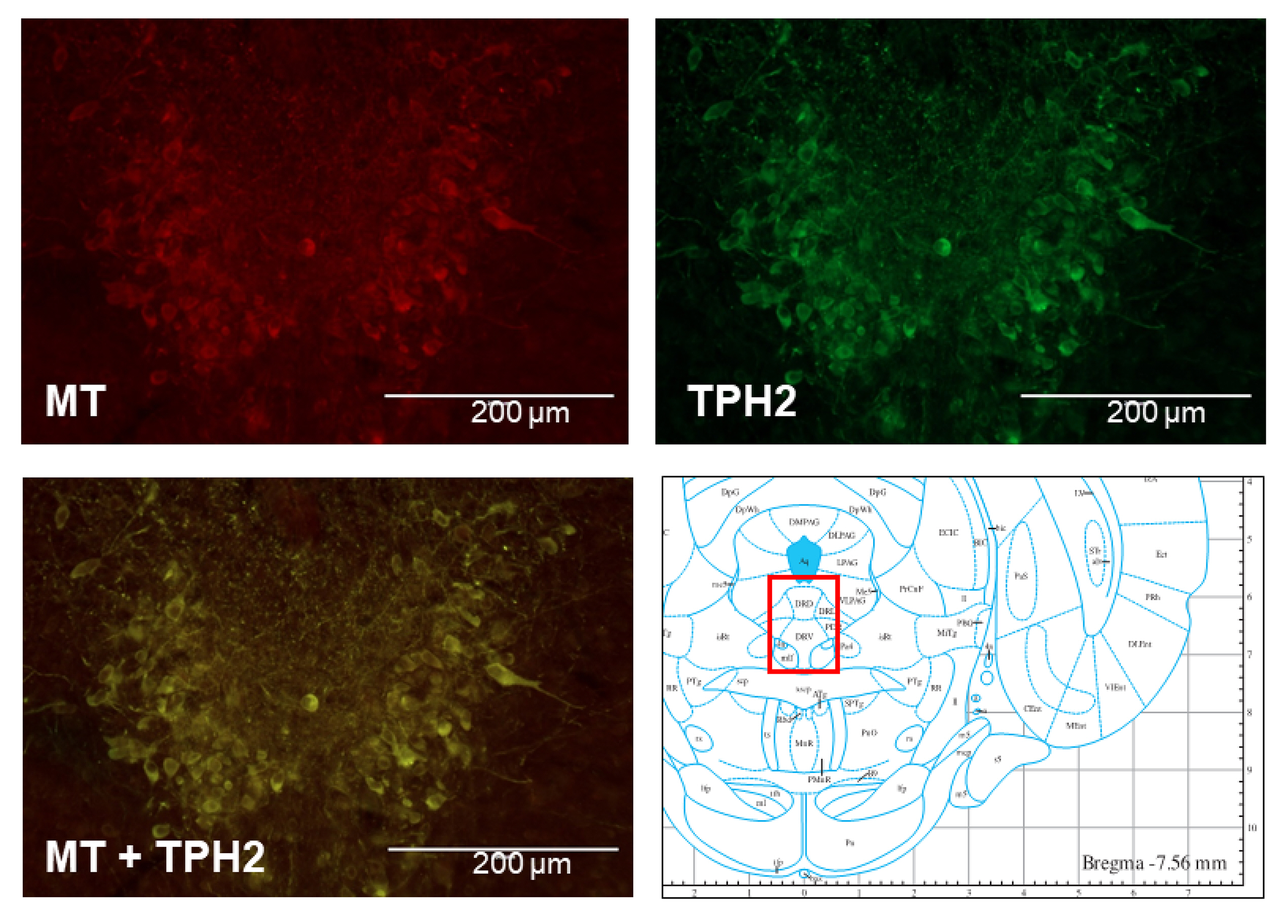
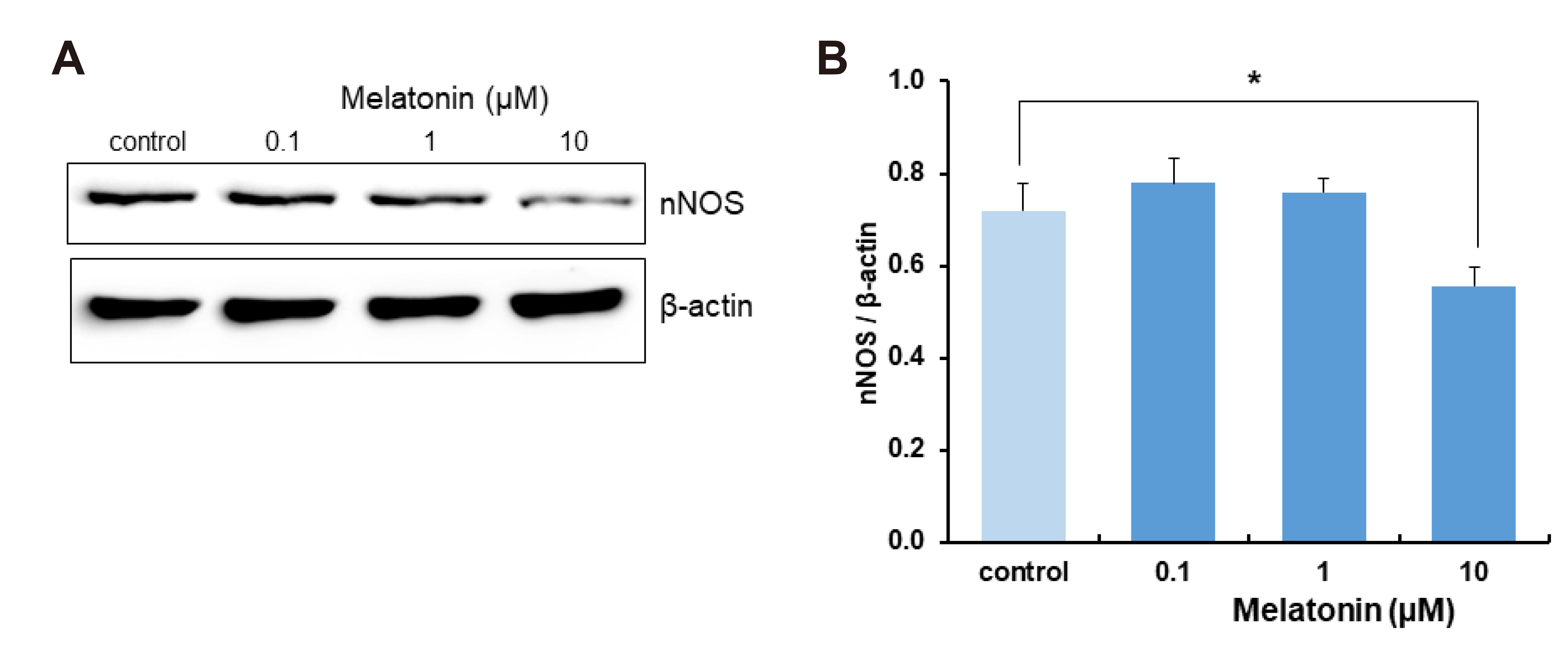
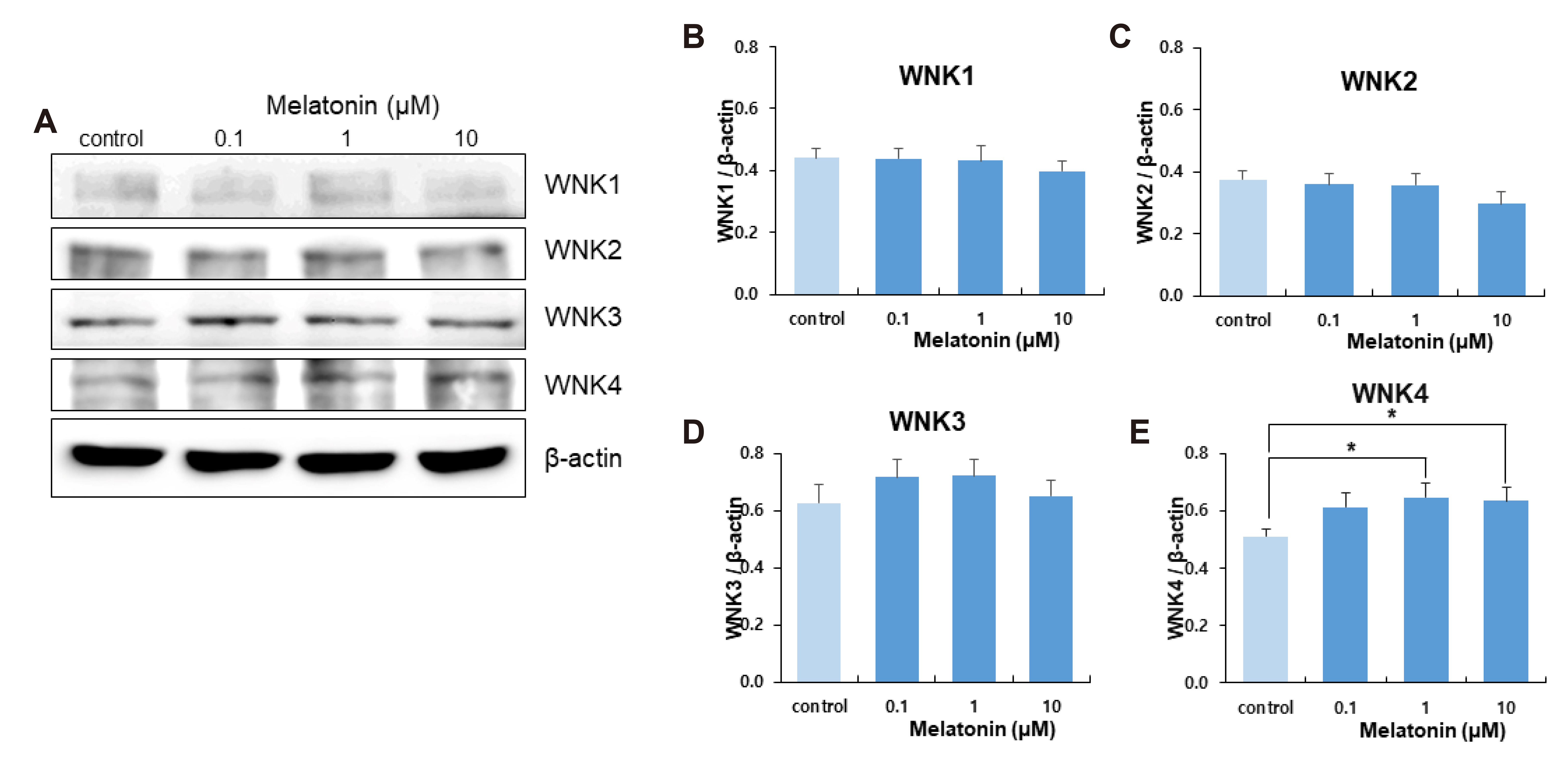
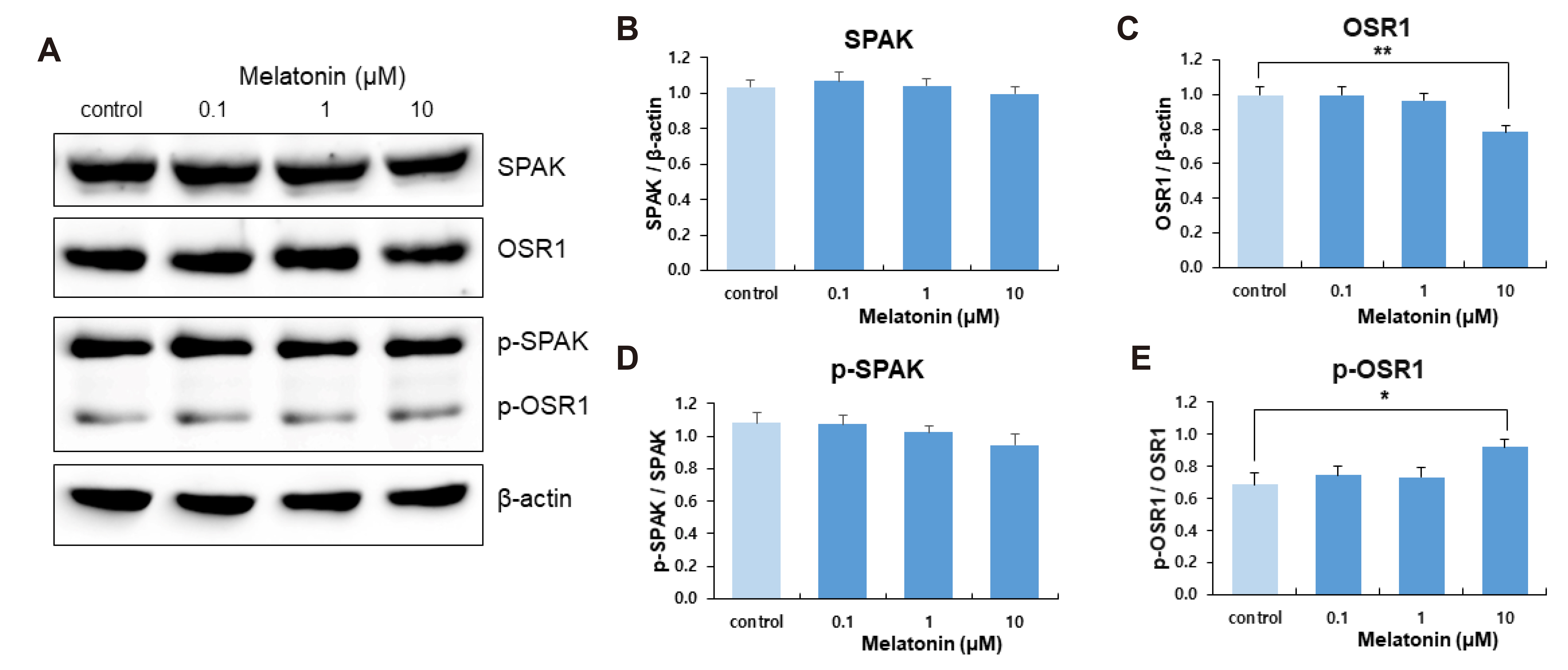
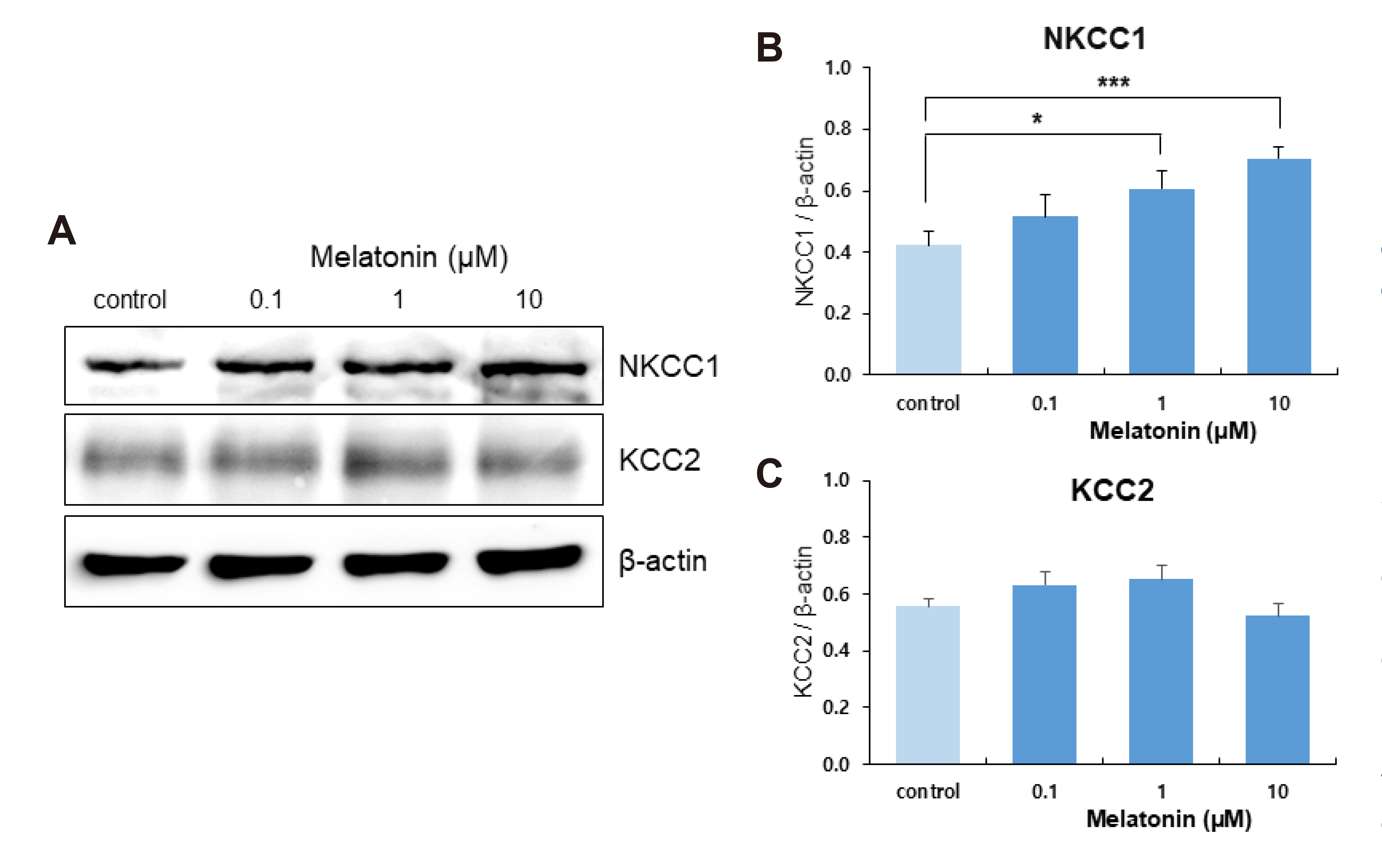
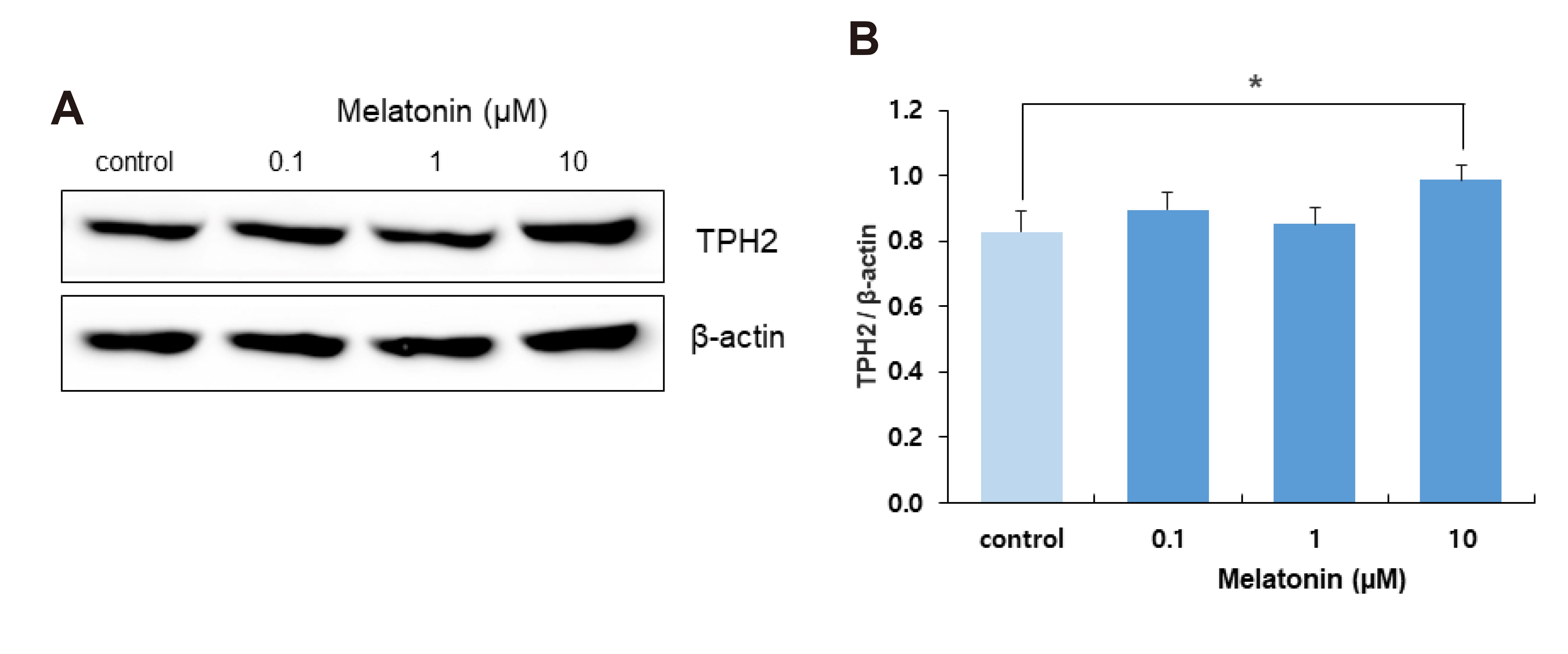
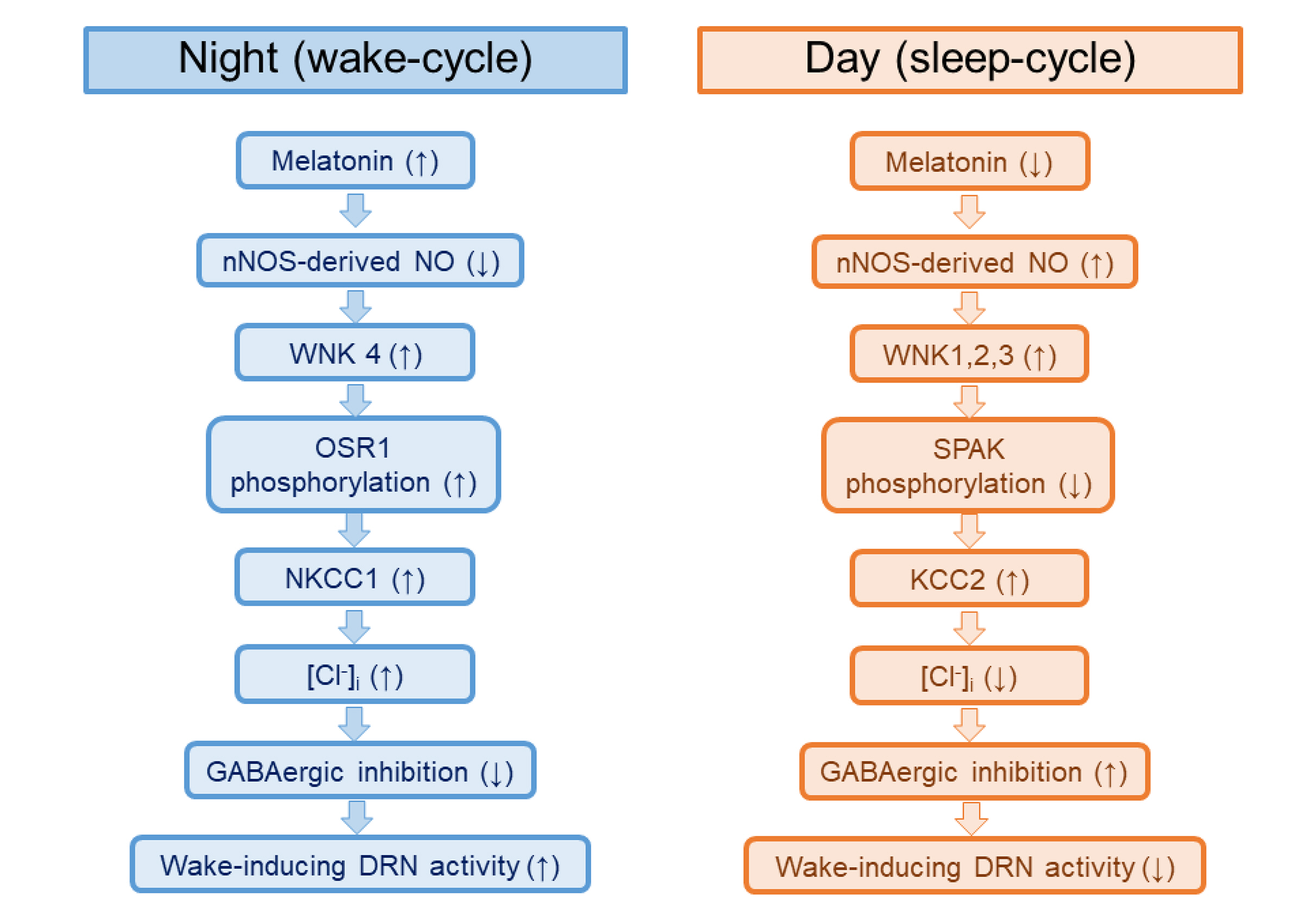
- The sleep-wake cycle is regulated by the alternating activity of sleep- and wake-promoting neurons. The dorsal raphe nucleus (DRN) secretes 5-hydroxytryptamine (5-HT, serotonin), promoting wakefulness. Melatonin secreted from the pineal gland also promotes wakefulness in rats. Our laboratory recently demonstrated that daily changes in nitric oxide (NO) production regulates a signaling pathway involving with-no-lysine kinase (WNK), Ste20-related proline alanine rich kinase (SPAK)/oxidative stress response kinase 1 (OSR1), and cation-chloride co-transporters (CCC) in rat DRN serotonergic neurons. This study was designed to investigate the effect of melatonin on NO-regulated WNK-SPAK/OSR1-CCC signaling in wake-inducing DRN neurons to elucidate the mechanism underlying melatonin’s wake-promoting actions in rats. Ex vivo treatment of DRN slices with melatonin suppressed neuronal nitric oxide synthase (nNOS) expression and increased WNK4 expression without altering WNK1, 2, or 3. Melatonin increased phosphorylation of OSR1 and the expression of sodium-potassium-chloride co-transporter 1 (NKCC1), while potassium-chloride cotransporter 2 (KCC2) remained unchanged. Melatonin increased the expression of tryptophan hydroxylase 2 (TPH2, serotonin-synthesizing enzyme). The present study suggests that melatonin may promote its wakefulness by modulating NO-regulated WNK-SPAK/OSR1-KNCC1 signaling in rat DRN serotonergic neurons.
Figure
Reference
-
1. Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O'Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M. 2013; Sleep drives metabolite clearance from the adult brain. Science. 342:373–377. DOI: 10.1126/science.1241224. PMID: 24136970. PMCID: PMC3880190.
Article2. Goldstein AN, Walker MP. 2014; The role of sleep in emotional brain function. Annu Rev Clin Psychol. 10:679–708. DOI: 10.1146/annurev-clinpsy-032813-153716. PMID: 24499013. PMCID: PMC4286245.
Article3. Vyazovskiy VV, Harris KD. 2013; Sleep and the single neuron: the role of global slow oscillations in individual cell rest. Nat Rev Neurosci. 14:443–451. DOI: 10.1038/nrn3494. PMID: 23635871. PMCID: PMC3972489.
Article4. Fuller PM, Gooley JJ, Saper CB. 2006; Neurobiology of the sleep-wake cycle: sleep architecture, circadian regulation, and regulatory feedback. J Biol Rhythms. 21:482–493. DOI: 10.1177/0748730406294627. PMID: 17107938.
Article5. Gallopin T, Fort P, Eggermann E, Cauli B, Luppi PH, Rossier J, Audinat E, Mühlethaler M, Serafin M. 2000; Identification of sleep-promoting neurons in vitro. Nature. 404:992–995. DOI: 10.1038/35010109. PMID: 10801127.
Article6. Lu J, Bjorkum AA, Xu M, Gaus SE, Shiromani PJ, Saper CB. 2002; Selective activation of the extended ventrolateral preoptic nucleus during rapid eye movement sleep. J Neurosci. 22:4568–4576. DOI: 10.1523/JNEUROSCI.22-11-04568.2002. PMID: 12040064. PMCID: PMC6758802.
Article7. Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. 2012; Control of sleep and wakefulness. Physiol Rev. 92:1087–1187. DOI: 10.1152/physrev.00032.2011. PMID: 22811426. PMCID: PMC3621793.
Article8. Sakai K. 2011; Sleep-waking discharge profiles of dorsal raphe nucleus neurons in mice. Neuroscience. 197:200–224. DOI: 10.1016/j.neuroscience.2011.09.024. PMID: 21958868.
Article9. Monti JM. 2010; The role of dorsal raphe nucleus serotonergic and non-serotonergic neurons, and of their receptors, in regulating waking and rapid eye movement (REM) sleep. Sleep Med Rev. 14:319–327. DOI: 10.1016/j.smrv.2009.10.003. PMID: 20153670.
Article10. Monti JM, Jantos H. 2008; The roles of dopamine and serotonin, and of their receptors, in regulating sleep and waking. Prog Brain Res. 172:625–646. DOI: 10.1016/S0079-6123(08)00929-1. PMID: 18772053.
Article11. Gervasoni D, Peyron C, Rampon C, Barbagli B, Chouvet G, Urbain N, Fort P, Luppi PH. 2000; Role and origin of the GABAergic innervation of dorsal raphe serotonergic neurons. J Neurosci. 20:4217–4225. DOI: 10.1523/JNEUROSCI.20-11-04217.2000. PMID: 10818157. PMCID: PMC6772634.
Article12. Payne JA, Rivera C, Voipio J, Kaila K. 2003; Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci. 26:199–206. DOI: 10.1016/S0166-2236(03)00068-7. PMID: 12689771.
Article13. Kaila K. 1994; Ionic basis of GABAA receptor channel function in the nervous system. Prog Neurobiol. 42:489–537. DOI: 10.1016/0301-0082(94)90049-3. PMID: 7522334.
Article14. Kaila K, Price TJ, Payne JA, Puskarjov M, Voipio J. 2014; Cation-chloride cotransporters in neuronal development, plasticity and disease. Nat Rev Neurosci. 15:637–654. DOI: 10.1038/nrn3819. PMID: 25234263. PMCID: PMC4294553.
Article15. Blaesse P, Airaksinen MS, Rivera C, Kaila K. 2009; Cation-chloride cotransporters and neuronal function. Neuron. 61:820–838. DOI: 10.1016/j.neuron.2009.03.003. PMID: 19323993.
Article16. Alessi DR, Zhang J, Khanna A, Hochdörfer T, Shang Y, Kahle KT. 2014; The WNK-SPAK/OSR1 pathway: master regulator of cation-chloride cotransporters. Sci Signal. 7:re3. DOI: 10.1126/scisignal.2005365. PMID: 25028718.
Article17. Kahle KT, Rinehart J, Lifton RP. 2010; Phosphoregulation of the Na-K-2Cl and K-Cl cotransporters by the WNK kinases. Biochim Biophys Acta. 1802:1150–1158. DOI: 10.1016/j.bbadis.2010.07.009. PMID: 20637866. PMCID: PMC3529164.
Article18. Piechotta K, Lu J, Delpire E. 2002; Cation chloride cotransporters interact with the stress-related kinases Ste20-related proline-alanine-rich kinase (SPAK) and oxidative stress response 1 (OSR1). J Biol Chem. 277:50812–50819. DOI: 10.1074/jbc.M208108200. PMID: 12386165.
Article19. Thastrup JO, Rafiqi FH, Vitari AC, Pozo-Guisado E, Deak M, Mehellou Y, Alessi DR. 2012; SPAK/OSR1 regulate NKCC1 and WNK activity: analysis of WNK isoform interactions and activation by T-loop trans-autophosphorylation. Biochem J. 441:325–337. DOI: 10.1042/BJ20111879. PMID: 22032326. PMCID: PMC3242505.
Article20. Ayers NA, Kapás L, Krueger JM. 1996; Circadian variation of nitric oxide synthase activity and cytosolic protein levels in rat brain. Brain Res. 707:127–130. DOI: 10.1016/0006-8993(95)01362-8. PMID: 8866722.
Article21. Chen L, Majde JA, Krueger JM. 2003; Spontaneous sleep in mice with targeted disruptions of neuronal or inducible nitric oxide synthase genes. Brain Res. 973:214–222. DOI: 10.1016/S0006-8993(03)02484-3. PMID: 12738065.
Article22. Kalinchuk AV, Stenberg D, Rosenberg PA, Porkka-Heiskanen T. 2006; Inducible and neuronal nitric oxide synthases (NOS) have complementary roles in recovery sleep induction. Eur J Neurosci. 24:1443–1456. DOI: 10.1111/j.1460-9568.2006.05019.x. PMID: 16987226.
Article23. Cespuglio R, Amrouni D, Meiller A, Buguet A, Gautier-Sauvigné S. 2012; Nitric oxide in the regulation of the sleep-wake states. Sleep Med Rev. 16:265–279. DOI: 10.1016/j.smrv.2012.01.006. PMID: 22406306.
Article24. Kim MJ, Yang HJ, Kim Y, Kang I, Kim SS, Cho YW. 2018; Role of nitric oxide and WNK-SPAK/OSR1-KCC2 signaling in daily changes in GABAergic inhibition in the rat dorsal raphe neurons. Neuropharmacology. 135:355–367. DOI: 10.1016/j.neuropharm.2018.03.035. PMID: 29596900.
Article25. Gandhi AV, Mosser EA, Oikonomou G, Prober DA. 2015; Melatonin is required for the circadian regulation of sleep. Neuron. 85:1193–1199. DOI: 10.1016/j.neuron.2015.02.016. PMID: 25754820. PMCID: PMC4851458.
Article26. Tan DX, Manchester LC, Esteban-Zubero E, Zhou Z, Reiter RJ. 2015; Melatonin as a potent and inducible endogenous antioxidant: synthesis and metabolism. Molecules. 20:18886–18906. DOI: 10.3390/molecules201018886. PMID: 26501252. PMCID: PMC6332205.
Article27. Szabadi E. 2006; Drugs for sleep disorders: mechanisms and therapeutic prospects. Br J Clin Pharmacol. 61:761–766. DOI: 10.1111/j.1365-2125.2006.02680.x. PMID: 16722842. PMCID: PMC1885116.
Article28. Schwartz MD, Wotus C, Liu T, Friesen WO, Borjigin J, Oda GA, de la Iglesia HO. 2009; Dissociation of circadian and light inhibition of melatonin release through forced desynchronization in the rat. Proc Natl Acad Sci U S A. 106:17540–17545. DOI: 10.1073/pnas.0906382106. PMID: 19805128. PMCID: PMC2762670.
Article29. Cajochen C, Kräuchi K, Wirz-Justice A. 2003; Role of melatonin in the regulation of human circadian rhythms and sleep. J Neuroendocrinol. 15:432–437. DOI: 10.1046/j.1365-2826.2003.00989.x. PMID: 12622846.
Article30. Descamps A, Rousset C, Millan MJ, Spedding M, Delagrange P, Cespuglio R. 2009; Influence of the novel antidepressant and melatonin agonist/serotonin2C receptor antagonist, agomelatine, on the rat sleep-wake cycle architecture. Psychopharmacology (Berl). 205:93–106. DOI: 10.1007/s00213-009-1519-2. PMID: 19370342.
Article31. Pevet P, Challet E. 2011; Melatonin: both master clock output and internal time-giver in the circadian clocks network. J Physiol Paris. 105:170–182. DOI: 10.1016/j.jphysparis.2011.07.001. PMID: 21914478.
Article32. Vriend J, Reiter RJ. 2015; Melatonin feedback on clock genes: a theory involving the proteasome. J Pineal Res. 58:1–11. DOI: 10.1111/jpi.12189. PMID: 25369242.
Article33. von Gall C, Stehle JH, Weaver DR. 2002; Mammalian melatonin receptors: molecular biology and signal transduction. Cell Tissue Res. 309:151–162. DOI: 10.1007/s00441-002-0581-4. PMID: 12111545.
Article34. Weaver DR, Reppert SM. 1996; The Mel1a melatonin receptor gene is expressed in human suprachiasmatic nuclei. Neuroreport. 8:109–112. DOI: 10.1097/00001756-199612200-00022. PMID: 9051762.
Article35. Kim JS, Kim WB, Kim YB, Lee Y, Kim YS, Shen FY, Lee SW, Park D, Choi HJ, Hur J, Park JJ, Han HC, Colwell CS, Cho YW, Kim YI. 2011; Chronic hyperosmotic stress converts GABAergic inhibition into excitation in vasopressin and oxytocin neurons in the rat. J Neurosci. 31:13312–13322. DOI: 10.1523/JNEUROSCI.1440-11.2011. PMID: 21917814. PMCID: PMC6623275.
Article36. Ruusuvirta T, Lipponen A, Pellinen EK, Penttonen M, Astikainen P. 2015; Auditory cortical and hippocampal local-field potentials to frequency deviant tones in urethane-anesthetized rats: an unexpected role of the sound frequencies themselves. Int J Psychophysiol. 96:134–140. DOI: 10.1016/j.ijpsycho.2015.04.007. PMID: 25911953.
Article37. Paxinos G, Watson C. 2007. The rat brain in stereotaxic coordinates. Academic Press;Cambridge:38. Hur J, Lee P, Kim MJ, Cho YW. 2014; Regulatory effect of 25-hydroxyvitamin D3 on nitric oxide production in activated microglia. Korean J Physiol Pharmacol. 18:397–402. DOI: 10.4196/kjpp.2014.18.5.397. PMID: 25352759. PMCID: PMC4211123.39. Arese M, Magnifico MC, Mastronicola D, Altieri F, Grillo C, Blanck TJ, Sarti P. 2012; Nanomolar melatonin enhances nNOS expression and controls HaCaT-cells bioenergetics. IUBMB Life. 64:251–258. DOI: 10.1002/iub.603. PMID: 22271455.
Article40. Gagnon KB, England R, Delpire E. 2006; Volume sensitivity of cation-Cl-cotransporters is modulated by the interaction of two kinases: Ste20-related proline-alanine-rich kinase and WNK4. Am J Physiol Cell Physiol. 290:C134–C142. DOI: 10.1152/ajpcell.00037.2005. PMID: 15930150.
Article41. de Los Heros P, Kahle KT, Rinehart J, Bobadilla NA, Vázquez N, San Cristobal P, Mount DB, Lifton RP, Hebert SC, Gamba G. 2006; WNK3 bypasses the tonicity requirement for K-Cl cotransporter activation via a phosphatase-dependent pathway. Proc Natl Acad Sci U S A. 103:1976–1981. DOI: 10.1073/pnas.0510947103. PMID: 16446421. PMCID: PMC1413675.
Article42. Rinehart J, Maksimova YD, Tanis JE, Stone KL, Hodson CA, Zhang J, Risinger M, Pan W, Wu D, Colangelo CM, Forbush B, Joiner CH, Gulcicek EE, Gallagher PG, Lifton RP. 2009; Sites of regulated phosphorylation that control K-Cl cotransporter activity. Cell. 138:525–536. DOI: 10.1016/j.cell.2009.05.031. PMID: 19665974. PMCID: PMC2811214.
Article43. Sarti P, Magnifico MC, Altieri F, Mastronicola D, Arese M. 2013; New evidence for cross talk between melatonin and mitochondria mediated by a circadian-compatible interaction with nitric oxide. Int J Mol Sci. 14:11259–11276. DOI: 10.3390/ijms140611259. PMID: 23759982. PMCID: PMC3709731.
Article44. Kahle KT, Rinehart J, Ring A, Gimenez I, Gamba G, Hebert SC, Lifton RP. 2006; WNK protein kinases modulate cellular Cl-flux by altering the phosphorylation state of the Na-K-Cl and K-Cl cotransporters. Physiology (Bethesda). 21:326–335. DOI: 10.1152/physiol.00015.2006. PMID: 16990453.45. Kahle KT, Deeb TZ, Puskarjov M, Silayeva L, Liang B, Kaila K, Moss SJ. 2013; Modulation of neuronal activity by phosphorylation of the K-Cl cotransporter KCC2. Trends Neurosci. 36:726–737. DOI: 10.1016/j.tins.2013.08.006. PMID: 24139641. PMCID: PMC4381966.
Article46. Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. 1999; The K+/Cl-co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 397:251–255. DOI: 10.1038/16697. PMID: 9930699.47. Monti JM. 2011; Serotonin control of sleep-wake behavior. Sleep Med Rev. 15:269–281. DOI: 10.1016/j.smrv.2010.11.003. PMID: 21459634.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ca2+ is a Regulator of the WNK/OSR1/NKCC Pathway in a Human Salivary Gland Cell Line
- The Effect of Melatonin Injection into Rat Brain Stem with Chronic Stress on Serotonergic Immunoreactivity
- The Effect of Photoperiod and Melatonin Injection on Serotonergic Immunoreactivity in Rat Brain Stem
- Immunohistochemical Study on the Nitric Oxide Synthase Neurons in the Brain Stem of Rats
- Elevated Serotonin-immunoreactive Neurons in the Raphe Nucleus of the Ataxic Mutant Mouse, Pogo