Korean J Physiol Pharmacol.
2015 May;19(3):249-255. 10.4196/kjpp.2015.19.3.249.
Ca2+ is a Regulator of the WNK/OSR1/NKCC Pathway in a Human Salivary Gland Cell Line
- Affiliations
-
- 1Department of Oral Biology, BK21 PLUS Project, Yonsei University College of Dentistry, Seoul 120-752, Korea. dmshin@yuhs.ac
- 2Department of Oral Medicine, Yonsei University College of Dentistry, Seoul 120-752, Korea. jhchoij@yuhs.ac
- KMID: 1791426
- DOI: http://doi.org/10.4196/kjpp.2015.19.3.249
Abstract
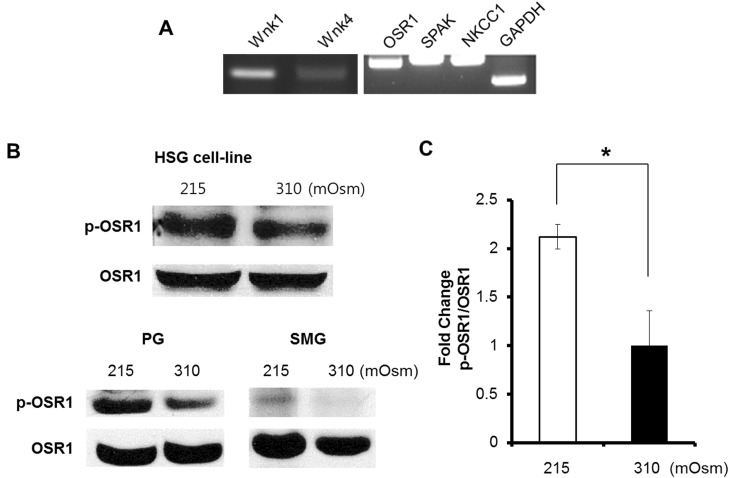
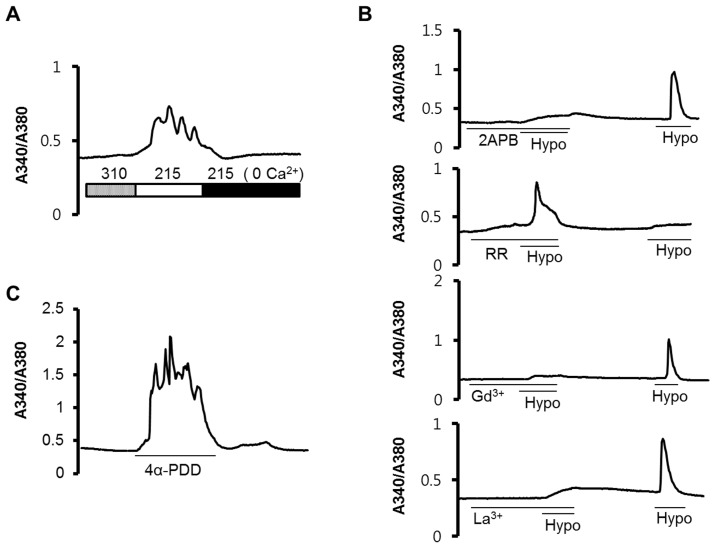
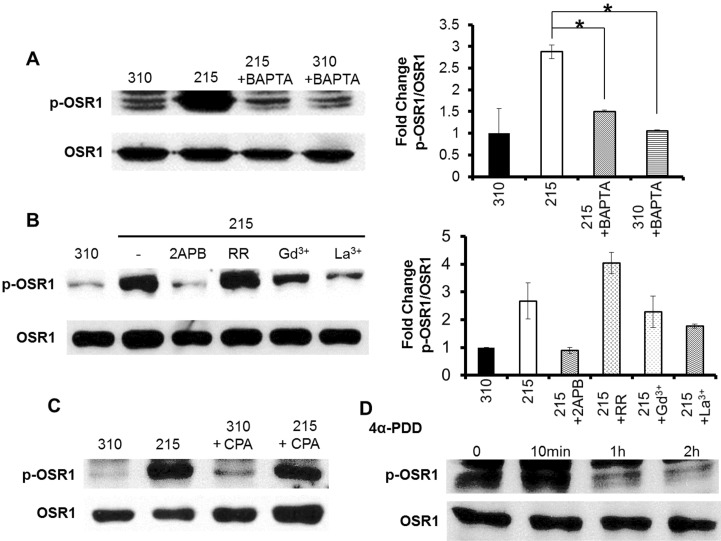
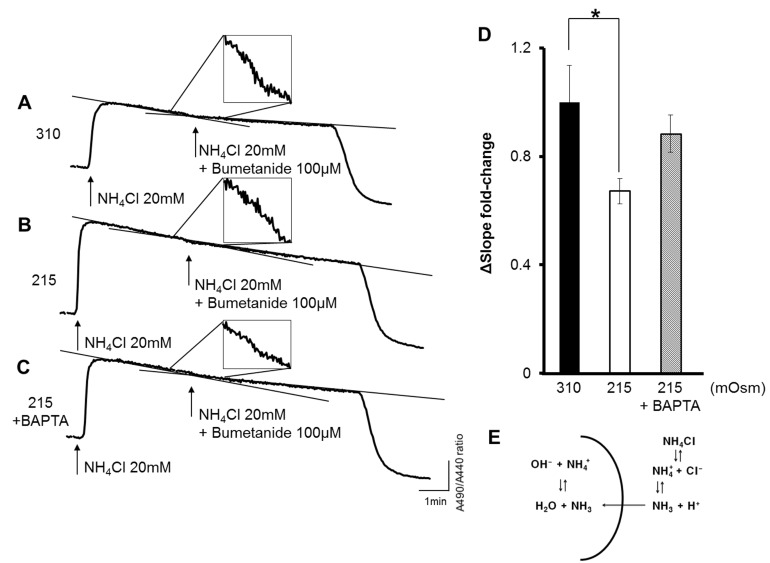
- Wnk kinase maintains cell volume, regulating various transporters such as sodium-chloride cotransporter, potassium-chloride cotransporter, and sodium-potassium-chloride cotransporter 1 (NKCC1) through the phosphorylation of oxidative stress responsive kinase 1 (OSR1) and STE20/SPS1-related proline/alanine-rich kinase (SPAK). However, the activating mechanism of Wnk kinase in specific tissues and specific conditions is broadly unclear. In the present study, we used a human salivary gland (HSG) cell line as a model and showed that Ca2+ may have a role in regulating Wnk kinase in the HSG cell line. Through this study, we found that the HSG cell line expressed molecules participating in the WNK-OSR1-NKCC pathway, such as Wnk1, Wnk4, OSR1, SPAK, and NKCC1. The HSG cell line showed an intracellular Ca2+ concentration ([Ca2+]i) increase in response to hypotonic stimulation, and the response was synchronized with the phosphorylation of OSR1. Interestingly, when we inhibited the hypotonically induced [Ca2+]i increase with nonspecific Ca2+ channel blockers such as 2-aminoethoxydiphenyl borate, gadolinium, and lanthanum, the phosphorylated OSR1 level was also diminished. Moreover, a cyclopiazonic acid-induced passive [Ca2+]i elevation was evoked by the phosphorylation of OSR1, and the amount of phosphorylated OSR1 decreased when the cells were treated with BAPTA, a Ca2+ chelator. Finally, through that process, NKCC1 activity also decreased to maintain the cell volume in the HSG cell line. These results indicate that Ca2+ may regulate the WNK-OSR1 pathway and NKCC1 activity in the HSG cell line. This is the first demonstration that indicates upstream Ca2+ regulation of the WNK-OSR1 pathway in intact cells.
Keyword
MeSH Terms
Figure
Reference
-
1. Xu B, English JM, Wilsbacher JL, Stippec S, Goldsmith EJ, Cobb MH. WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J Biol Chem. 2000; 275:16795–16801. PMID: 10828064.
Article2. Verissimo F, Jordan P. WNK kinases, a novel protein kinase subfamily in multi-cellular organisms. Oncogene. 2001; 20:5562–5569. PMID: 11571656.
Article3. Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science. 2001; 293:1107–1112. PMID: 11498583.
Article4. Gamba G. Role of WNK kinases in regulating tubular salt and potassium transport and in the development of hypertension. Am J Physiol Renal Physiol. 2005; 288:F245–F252. PMID: 15637347.
Article5. Kahle KT, Wilson FH, Leng Q, Lalioti MD, O'Connell AD, Dong K, Rapson AK, MacGregor GG, Giebisch G, Hebert SC, Lifton RP. WNK4 regulates the balance between renal NaCl reabsorption and K+ secretion. Nat Genet. 2003; 35:372–376. PMID: 14608358.6. Vitari AC, Deak M, Morrice NA, Alessi DR. The WNK1 and WNK4 protein kinases that are mutated in Gordon's hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases. Biochem J. 2005; 391:17–24. PMID: 16083423.
Article7. Rinehart J, Kahle KT, de Los, Vazquez N, Meade P, Wilson FH, Hebert SC, Gimenez I, Gamba G, Lifton RP. WNK3 kinase is a positive regulator of NKCC2 and NCC, renal cation-Cl-cotransporters required for normal blood pressure homeostasis. Proc Natl Acad Sci U S A. 2005; 102:16777–16782. PMID: 16275913.8. Moriguchi T, Urushiyama S, Hisamoto N, Iemura S, Uchida S, Natsume T, Matsumoto K, Shibuya H. WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J Biol Chem. 2005; 280:42685–42693. PMID: 16263722.
Article9. Kahle KT, Macgregor GG, Wilson FH, Van Hoek AN, Brown D, Ardito T, Kashgarian M, Giebisch G, Hebert SC, Boulpaep EL, Lifton RP. Paracellular Cl- permeability is regulated by WNK4 kinase: insight into normal physiology and hypertension. Proc Natl Acad Sci U S A. 2004; 101:14877–14882. PMID: 15465913.
Article10. Yamauchi K, Rai T, Kobayashi K, Sohara E, Suzuki T, Itoh T, Suda S, Hayama A, Sasaki S, Uchida S. Disease-causing mutant WNK4 increases paracellular chloride permeability and phosphorylates claudins. Proc Natl Acad Sci U S A. 2004; 101:4690–4694. PMID: 15070779.
Article11. Xu BE, Min X, Stippec S, Lee BH, Goldsmith EJ, Cobb MH. Regulation of WNK1 by an auto-inhibitory domain and autophosphorylation. J Biol Chem. 2002; 277:48456–48462. PMID: 12374799.
Article12. Lenertz LY, Lee BH, Min X, Xu BE, Wedin K, Earnest S, Goldsmith EJ, Cobb MH. Properties of WNK1 and implications for other family members. J Biol Chem. 2005; 280:26653–26658. PMID: 15883153.
Article13. Alessi DR, Zhang J, Khanna A, Hochdorfer T, Shang Y, Kahle KT. The WNK-SPAK/OSR1 pathway: master regulator of cation-chloride cotransporters. Sci Signal. 2014; 7:re3. PMID: 25028718.
Article14. Arroyo JP, Kahle KT, Gamba G. The SLC12 family of electroneutral cation-coupled chloride cotransporters. Mol Aspects Med. 2013; 34:288–298. PMID: 23506871.
Article15. Kahle KT, Rinehart J, Lifton RP. Phosphoregulation of the Na-K-2Cl and K-Cl cotransporters by the WNK kinases. Biochim Biophys Acta. 2010; 1802:1150–1158. PMID: 20637866.
Article16. Schumacher FR, Sorrell FJ, Alessi DR, Bullock AN, Kurz T. Structural and biochemical characterization of the KLHL3-WNK kinase interaction important in blood pressure regulation. Biochem J. 2014; 460:237–246. PMID: 24641320.
Article17. McCormick JA, Yang CL, Zhang C, Davidge B, Blankenstein KI, Terker AS, Yarbrough B, Meermeier NP, Park HJ, McCully B, West M, Borschewski A, Himmerkus N, Bleich M, Bachmann S, Mutig K, Argaiz ER, Gamba G, Singer JD, Ellison DH. Hyperkalemic hypertension-associated cullin 3 promotes WNK signaling by degrading KLHL3. J Clin Invest. 2014; 124:4723–4736. PMID: 25250572.
Article18. Wakabayashi M, Mori T, Isobe K, Sohara E, Susa K, Araki Y, Chiga M, Kikuchi E, Nomura N, Mori Y, Matsuo H, Murata T, Nomura S, Asano T, Kawaguchi H, Nonoyama S, Rai T, Sasaki S, Uchida S. Impaired KLHL3-mediated ubiquitination of WNK4 causes human hypertension. Cell Rep. 2013; 3:858–868. PMID: 23453970.
Article19. Takahashi D, Mori T, Wakabayashi M, Mori Y, Susa K, Zeniya M, Sohara E, Rai T, Sasaki S, Uchida S. KLHL2 interacts with and ubiquitinates WNK kinases. Biochem Biophys Res Commun. 2013; 437:457–462. PMID: 23838290.
Article20. Ohta A, Schumacher FR, Mehellou Y, Johnson C, Knebel A, Macartney TJ, Wood NT, Alessi DR, Kurz T. The CUL3-KLHL3 E3 ligase complex mutated in Gordon's hypertension syndrome interacts with and ubiquitylates WNK isoforms: disease-causing mutations in KLHL3 and WNK4 disrupt interaction. Biochem J. 2013; 451:111–122. PMID: 23387299.
Article21. Mori Y, Wakabayashi M, Mori T, Araki Y, Sohara E, Rai T, Sasaki S, Uchida S. Decrease of WNK4 ubiquitination by disease-causing mutations of KLHL3 through different molecular mechanisms. Biochem Biophys Res Commun. 2013; 439:30–34. PMID: 23962426.
Article22. Hossain Khan MZ, Sohara E, Ohta A, Chiga M, Inoue Y, Isobe K, Wakabayashi M, Oi K, Rai T, Sasaki S, Uchida S. Phosphorylation of Na-Cl cotransporter by OSR1 and SPAK kinases regulates its ubiquitination. Biochem Biophys Res Commun. 2012; 425:456–461. PMID: 22846565.
Article23. Nishida H, Sohara E, Nomura N, Chiga M, Alessi DR, Rai T, Sasaki S, Uchida S. Phosphatidylinositol 3-kinase/Akt signaling pathway activates the WNK-OSR1/SPAK-NCC phosphorylation cascade in hyperinsulinemic db/db mice. Hypertension. 2012; 60:981–990. PMID: 22949526.
Article24. Naguro I, Umeda T, Kobayashi Y, Maruyama J, Hattori K, Shimizu Y, Kataoka K, Kim-Mitsuyama S, Uchida S, Vandewalle A, Noguchi T, Nishitoh H, Matsuzawa A, Takeda K, Ichijo H. ASK3 responds to osmotic stress and regulates blood pressure by suppressing WNK1-SPAK/OSR1 signaling in the kidney. Nat Commun. 2012; 3:1285. PMID: 23250415.
Article25. Kaji T, Yoshida S, Kawai K, Fuchigami Y, Watanabe W, Kubodera H, Kishimoto T. ASK3, a novel member of the apoptosis signal-regulating kinase family, is essential for stress-induced cell death in HeLa cells. Biochem Biophys Res Commun. 2010; 395:213–218. PMID: 20362554.
Article26. Dowd BF, Forbush B. PASK (proline-alanine-rich STE20-related kinase), a regulatory kinase of the Na-K-Cl cotransporter (NKCC1). J Biol Chem. 2003; 278:27347–27353. PMID: 12740379.
Article27. Piala AT, Moon TM, Akella R, He H, Cobb MH, Goldsmith EJ. Chloride sensing by WNK1 involves inhibition of autophosphorylation. Sci Signal. 2014; 7:ra41. PMID: 24803536.
Article28. Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003; 4:517–529. PMID: 12838335.
Article29. Park S, Lee SI, Shin DM. Role of regulators of g-protein signaling 4 in ca signaling in mouse pancreatic acinar cells. Korean J Physiol Pharmacol. 2011; 15:383–388. PMID: 22359476.30. Evans RL, Turner RJ. Upregulation of Na(+)-K(+)-2Cl- cotransporter activity in rat parotid acinar cells by muscarinic stimulation. J Physiol. 1997; 499:351–359. PMID: 9080365.
Article31. Na T, Wu G, Peng JB. Disease-causing mutations in the acidic motif of WNK4 impair the sensitivity of WNK4 kinase to calcium ions. Biochem Biophys Res Commun. 2012; 419:293–298. PMID: 22342722.
Article32. Hoorn EJ, Walsh SB, McCormick JA, Furstenberg A, Yang CL, Roeschel T, Paliege A, Howie AJ, Conley J, Bachmann S, Unwin RJ, Ellison DH. The calcineurin inhibitor tacrolimus activates the renal sodium chloride cotransporter to cause hypertension. Nat Med. 2011; 17:1304–1309. PMID: 21963515.
Article33. Diver JM, Sage SO, Rosado JA. The inositol trisphosphate receptor antagonist 2-aminoethoxydiphenylborate (2-APB) blocks Ca2+ entry channels in human platelets: cautions for its use in studying Ca2+ influx. Cell Calcium. 2001; 30:323–329. PMID: 11733938.34. Bootman MD, Collins TJ, Mackenzie L, Roderick HL, Berridge MJ, Peppiatt CM. 2-aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J. 2002; 16:1145–1150. PMID: 12153982.35. Hatano N, Itoh Y, Muraki K. Cardiac fibroblasts have functional TRPV4 activated by 4alpha-phorbol 12,13-didecanoate. Life Sci. 2009; 85:808–814. PMID: 19879881.36. Kim KS, Shin DH, Nam JH, Park KS, Zhang YH, Kim WK, Kim SJ. Functional expression of trpv4 cation channels in human mast cell line (HMC-1). Korean J Physiol Pharmacol. 2010; 14:419–425. PMID: 21311684.
Article37. Nilius B, Vriens J, Prenen J, Droogmans G, Voets T. TRPV4 calcium entry channel: a paradigm for gating diversity. Am J Physiol Cell Physiol. 2004; 286:C195–C205. PMID: 14707014.
Article38. Kline D, Kline JT. Repetitive calcium transients and the role of calcium in exocytosis and cell cycle activation in the mouse egg. Dev Biol. 1992; 149:80–89. PMID: 1728596.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Melatonin modulates nitric oxide-regulated WNK-SPAK/OSR1-NKCC1 signaling in dorsal raphe nucleus of rats
- TRPV1 in Salivary Gland Epithelial Cells Is Not Involved in Salivary Secretion via Transcellular Pathway
- DA-6034 Induces [Ca2+]i Increase in Epithelial Cells
- Differentiation and Characterization of Cystic Fibrosis Transmembrane Conductance Regulator Knockout Human Pluripotent Stem Cells into Salivary Gland Epithelial Progenitors
- Salivary Duct Carcinoma: 2 Case Reports