Korean J Physiol Pharmacol.
2021 Sep;25(5):425-437. 10.4196/kjpp.2021.25.5.425.
Sitagliptin attenuates endothelial dysfunction independent of its blood glucose controlling effect
- Affiliations
-
- 1Department of Endocrinology, Beijing Hospital, National Center of Gerontology, Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing 100730, P. R. China.
- 2Graduate School of Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing 100730, P. R. China.
- 3The Key Laboratory of Geriatrics, Beijing Institute of Geriatrics, Beijing Hospital, National Center of Gerontology, National Health Commission, Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing 100730, P. R. China.
- KMID: 2519405
- DOI: http://doi.org/10.4196/kjpp.2021.25.5.425
Abstract
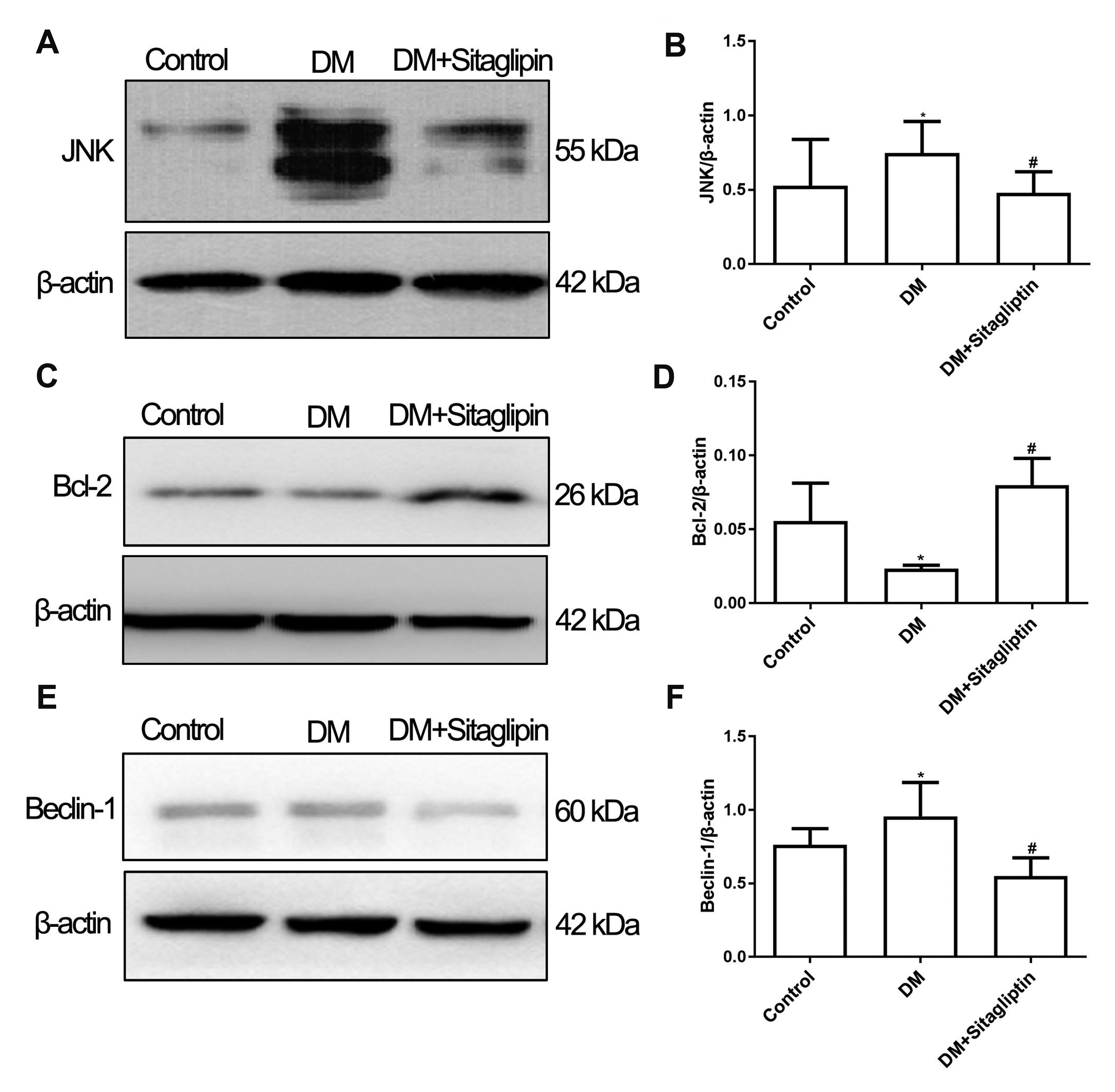
- Although the contributions of sitagliptin to endothelial dysfunction in diabetes mellitus were previously reported, the mechanisms still undefined. Autophagy plays an important role in the development of diabetes mellitus, but its role in diabetic macrovascular complications is unclear. This study aims to observe the effect of sitagliptin on macrovascular endothelium in diabetes and explore the role of autophagy in this process. Diabetic rats were induced through administration of high-fat diet and intraperitoneal injection of streptozotocin. Then diabetic rats were treated with or without sitagliptin for 12 weeks. Endothelial damage and autophagy were measured. Human umbilical vein endothelial cells were cultured either in normal glucose or in high glucose medium and intervened with different concentrations of sitagliptin. Rapamycin was used to induce autophagy. Cell viability, apoptosis and autophagy were detected. The expressions of proteins in c-Jun N-terminal kinase (JNK)-Bcl-2-Beclin-1 pathway were measured. Sitagliptin attenuated injuries of endothelium in vivo and in vitro. The expression of microtubuleassociated protein 1 light chain 3 II (LC3II) and beclin-1 were increased in aortas of diabetic rats and cells cultured with high-glucose, while sitagliptin inhibited the over-expression of LC3II and beclin-1. In vitro pre-treatment with sitagliptin decreased rapamycin-induced autophagy. However, after pretreatment with rapamycin, the protective effect of sitagliptin on endothelial cells was abolished. Further studies revealed sitagliptin increased the expression of Bcl-2, while inhibited the expression of JNK in vivo . Sitagliptin attenuates injuries of vascular endothelial cells caused by high glucose through inhibiting over-activated autophagy. JNK-Bcl-2-Beclin-1 pathway may be involved in this process.
Keyword
Figure
Cited by 1 articles
-
Experimental model and novel therapeutic targets for non-alcoholic fatty liver disease development
Yujin Jin, Kyung-Sun Heo
Korean J Physiol Pharmacol. 2023;27(4):299-310. doi: 10.4196/kjpp.2023.27.4.299.
Reference
-
1. An Y, Zhang P, Wang J, Gong Q, Gregg EW, Yang W, Li H, Zhang B, Shuai Y, Chen Y, Engelgau MM, Cheng Y, Hu Y, Bennett PH, Li G. 2015; Cardiovascular and all-cause mortality over a 23-year period among Chinese with newly diagnosed diabetes in the Da Qing IGT and diabetes study. Diabetes Care. 38:1365–1371. DOI: 10.2337/dc14-2498. PMID: 25887356. PMCID: PMC4477333.
Article2. Mora PF, Johnson EL. 2017; Cardiovascular outcome trials of the incretin-based therapies: what do we know so far? Endocr Pract. 23:89–99. DOI: 10.4158/EP161481.RA. PMID: 27819769.
Article3. Rizzo M, Rizvi AA, Spinas GA, Rini GB, Berneis K. 2009; Glucose lowering and anti-atherogenic effects of incretin-based therapies: GLP-1 analogues and DPP-4-inhibitors. Expert Opin Investig Drugs. 18:1495–1503. DOI: 10.1517/14728220903241633. PMID: 19758106.
Article4. Vilsbøll T, Garber AJ. 2012; Non-glycaemic effects mediated via GLP-1 receptor agonists and the potential for exploiting these for therapeutic benefit: focus on liraglutide. Diabetes Obes Metab. 14 Suppl 2:41–49. DOI: 10.1111/j.1463-1326.2012.01579.x. PMID: 22405268.
Article5. Ahrén B, Schmitz O. 2004; GLP-1 receptor agonists and DPP-4 inhibitors in the treatment of type 2 diabetes. Horm Metab Res. 36:867–876. DOI: 10.1055/s-2004-826178. PMID: 15655721.
Article6. Wu C, Hu S, Wang N, Tian J. 2017; Dipeptidyl peptidase-4 inhibitor sitagliptin prevents high glucose-induced apoptosis via activation of AMP-activated protein kinase in endothelial cells. Mol Med Rep. 15:4346–4351. DOI: 10.3892/mmr.2017.6501. PMID: 28440488.
Article7. Tang ST, Su H, Zhang Q, Tang HQ, Wang CJ, Zhou Q, Wei W, Zhu HQ, Wang Y. 2016; Sitagliptin inhibits endothelin-1 expression in the aortic endothelium of rats with streptozotocin-induced diabetes by suppressing the nuclear factor-κB/IκBα system through the activation of AMP-activated protein kinase. Int J Mol Med. 37:1558–1566. DOI: 10.3892/ijmm.2016.2578. PMID: 27122056. PMCID: PMC4866950.
Article8. Wang H, Zhou Y, Guo Z, Dong Y, Xu J, Huang H, Liu H, Wang W. 2018; Sitagliptin attenuates endothelial dysfunction of Zucker diabetic fatty rats: implication of the antiperoxynitrite and autophagy. J Cardiovasc Pharmacol Ther. 23:66–78. DOI: 10.1177/1074248417715001. PMID: 28618859.
Article9. Dai X, Zeng J, Yan X, Lin Q, Wang K, Chen J, Shen F, Gu X, Wang Y, Chen J, Pan K, Cai L, Wintergerst KA, Tan Y. 2018; Sitagliptin-mediated preservation of endothelial progenitor cell function via augmenting autophagy enhances ischaemic angiogenesis in diabetes. J Cell Mol Med. 22:89–100. DOI: 10.1111/jcmm.13296. PMID: 28799229. PMCID: PMC5742710.
Article10. Shao S, Xu Q, Yu X, Pan R, Chen Y. 2020; Dipeptidyl peptidase 4 inhibitors and their potential immune modulatory functions. Pharmacol Ther. 209:107503. DOI: 10.1016/j.pharmthera.2020.107503. PMID: 32061923. PMCID: PMC7102585.
Article11. Silva Júnior WS, Godoy-Matos AF, Kraemer-Aguiar LG. 2015; Dipeptidyl peptidase 4: a new link between diabetes mellitus and atherosclerosis? Biomed Res Int. 2015:816164. DOI: 10.1155/2015/816164. PMID: 26146634. PMCID: PMC4471315.
Article12. Price JD, Linder G, Li WP, Zimmermann B, Rother KI, Malek R, Alattar M, Tarbell KV. 2013; Effects of short-term sitagliptin treatment on immune parameters in healthy individuals, a randomized placebo-controlled study. Clin Exp Immunol. 174:120–128. DOI: 10.1111/cei.12144. PMID: 23711188. PMCID: PMC3784219.
Article13. Zilleßen P, Celner J, Kretschmann A, Pfeifer A, Racké K, Mayer P. 2016; Metabolic role of dipeptidyl peptidase 4 (DPP4) in primary human (pre)adipocytes. Sci Rep. 6:23074. DOI: 10.1038/srep23074. PMID: 26983599. PMCID: PMC4794806.
Article14. Graham ML, Janecek JL, Kittredge JA, Hering BJ, Schuurman HJ. 2011; The streptozotocin-induced diabetic nude mouse model: differences between animals from different sources. Comp Med. 61:356–360. PMID: 22330251. PMCID: PMC3155402.15. Baudin B, Bruneel A, Bosselut N, Vaubourdolle M. 2007; A protocol for isolation and culture of human umbilical vein endothelial cells. Nat Protoc. 2:481–485. DOI: 10.1038/nprot.2007.54. PMID: 17406610.
Article16. Goncharov NV, Nadeev AD, Jenkins RO, Avdonin PV. 2017; Markers and biomarkers of endothelium: when something is rotten in the state. Oxid Med Cell Longev. 2017:9759735. DOI: 10.1155/2017/9759735. PMID: 29333215. PMCID: PMC5733214.
Article17. Mizushima N, Yoshimori T. 2007; How to interpret LC3 immunoblotting. Autophagy. 3:542–545. DOI: 10.4161/auto.4600. PMID: 17611390.
Article18. Kang R, Zeh HJ, Lotze MT, Tang D. 2011; The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 18:571–580. DOI: 10.1038/cdd.2010.191. PMID: 21311563. PMCID: PMC3131912.
Article19. Wang XM, Yang YJ, Wu YJ, Zhang Q, Qian HY. 2015; Attenuating hypoxia-induced apoptosis and autophagy of mesenchymal stem cells: the potential of sitagliptin in stem cell-based therapy. Cell Physiol Biochem. 37:1914–1926. DOI: 10.1159/000438552. PMID: 26584290.
Article20. Liu L, Liu J, Tian XY, Wong WT, Lau CW, Xu A, Xu G, Ng CF, Yao X, Gao Y, Huang Y. 2014; Uncoupling protein-2 mediates DPP-4 inhibitor-induced restoration of endothelial function in hypertension through reducing oxidative stress. Antioxid Redox Signal. 21:1571–1581. DOI: 10.1089/ars.2013.5519. PMID: 24328731. PMCID: PMC4174421.
Article21. Zeng Y, Li C, Guan M, Zheng Z, Li J, Xu W, Wang L, He F, Xue Y. 2014; The DPP-4 inhibitor sitagliptin attenuates the progress of atherosclerosis in apolipoprotein-E-knockout mice via AMPK- and MAPK-dependent mechanisms. Cardiovasc Diabetol. 13:32. DOI: 10.1186/1475-2840-13-32. PMID: 24490809. PMCID: PMC3916068.
Article22. Levine B, Kroemer G. 2008; Autophagy in the pathogenesis of disease. Cell. 132:27–42. DOI: 10.1016/j.cell.2007.12.018. PMID: 18191218. PMCID: PMC2696814.
Article23. Cai X, She M, Xu M, Chen H, Li J, Chen X, Zheng D, Liu J, Chen S, Zhu J, Xu X, Li R, Li J, Chen S, Yang X, Li H. 2018; GLP-1 treatment protects endothelial cells from oxidative stress-induced autophagy and endothelial dysfunction. Int J Biol Sci. 14:1696–1708. DOI: 10.7150/ijbs.27774. PMID: 30416384. PMCID: PMC6216037.
Article24. Niu C, Chen Z, Kim KT, Sun J, Xue M, Chen G, Li S, Shen Y, Zhu Z, Wang X, Liang J, Jiang C, Cong W, Jin L, Li X. 2019; Metformin alleviates hyperglycemia-induced endothelial impairment by downregulating autophagy via the Hedgehog pathway. Autophagy. 15:843–870. DOI: 10.1080/15548627.2019.1569913. PMID: 30653446. PMCID: PMC6526809.
Article25. Chen F, Chen B, Xiao FQ, Wu YT, Wang RH, Sun ZW, Fu GS, Mou Y, Tao W, Hu XS, Hu SJ. 2014; Autophagy protects against senescence and apoptosis via the RAS-mitochondria in high-glucose-induced endothelial cells. Cell Physiol Biochem. 33:1058–1074. DOI: 10.1159/000358676. PMID: 24732710.
Article26. Chao CL, Chuang CP, Cheng YF, Lee KR, Chang Y, Cheng SP, Chan WK, Ho FM. 2016; The protective role of autophagy in matrix metalloproteinase-mediated cell transmigration and cell death in high-glucose-treated endothelial cells. Inflammation. 39:830–838. DOI: 10.1007/s10753-016-0313-7. PMID: 26846884.
Article27. Zhang J, Deng H, Liu L, Liu X, Zuo X, Xu Q, Wu Z, Peng X, Ji A. 2015; α-Lipoic acid protects against hypoxia/reoxygenation-induced injury in human umbilical vein endothelial cells through suppression of apoptosis and autophagy. Mol Med Rep. 12:180–186. DOI: 10.3892/mmr.2015.3351. PMID: 25684163. PMCID: PMC4438966.
Article28. Xu Q, Li X, Lu Y, Shen L, Zhang J, Cao S, Huang X, Bin J, Liao Y. 2015; Pharmacological modulation of autophagy to protect cardiomyocytes according to the time windows of ischaemia/reperfusion. Br J Pharmacol. 172:3072–3085. DOI: 10.1111/bph.13111. PMID: 25660104. PMCID: PMC4459024.
Article29. Mellor KM, Bell JR, Young MJ, Ritchie RH, Delbridge LM. 2011; Myocardial autophagy activation and suppressed survival signaling is associated with insulin resistance in fructose-fed mice. J Mol Cell Cardiol. 50:1035–1043. DOI: 10.1016/j.yjmcc.2011.03.002. PMID: 21385586.
Article30. Wu X, He L, Chen F, He X, Cai Y, Zhang G, Yi Q, He M, Luo J. 2014; Impaired autophagy contributes to adverse cardiac remodeling in acute myocardial infarction. PLoS One. 9:e112891. DOI: 10.1371/journal.pone.0112891. PMID: 25409294. PMCID: PMC4237367.
Article31. Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A. 2009; Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ. 16:966–975. DOI: 10.1038/cdd.2009.33. PMID: 19325568.
Article32. Luo S, Rubinsztein DC. 2013; BCL2L11/BIM: a novel molecular link between autophagy and apoptosis. Autophagy. 9:104–105. DOI: 10.4161/auto.22399. PMID: 23064249. PMCID: PMC3542209.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Combined effects of synbiotic and sitagliptin versus sitagliptin alone in patients with nonalcoholic fatty liver disease
- Combination of synbiotic and sitagliptin in nonalcoholic fatty liver disease: Is it better than sitagliptin alone?
- Factors Influencing Glycemic Control Response of Sitagliptin
- Efficacy of Sitagliptin When Added to Ongoing Therapy in Korean Subjects with Type 2 Diabetes Mellitus
- Can We Restore the Endothelial Dysfunction in Patients with Essential Hypertension with Calcium Channel Blockers?