J Korean Med Sci.
2021 Aug;36(31):e198. 10.3346/jkms.2021.36.e198.
Machine Learning Approach for Active Vaccine Safety Monitoring
- Affiliations
-
- 1Department of Biomedical Systems Informatics, Yonsei University College of Medicine, Yongin, Korea
- 2Department of Biomedical Informatics, Ajou University School of Medicine, Suwon, Korea
- 3Department of Health Convergence, Ewha Womans University, Seoul, Korea
- 4Center for Digital Health, Yongin Severance Hospital, Yonsei University Health System, Yongin, Korea
- KMID: 2519199
- DOI: http://doi.org/10.3346/jkms.2021.36.e198
Abstract
- Background
Vaccine safety surveillance is important because it is related to vaccine hesitancy, which affects vaccination rate. To increase confidence in vaccination, the active monitoring of vaccine adverse events is important. For effective active surveillance, we developed and verified a machine learning-based active surveillance system using national claim data.
Methods
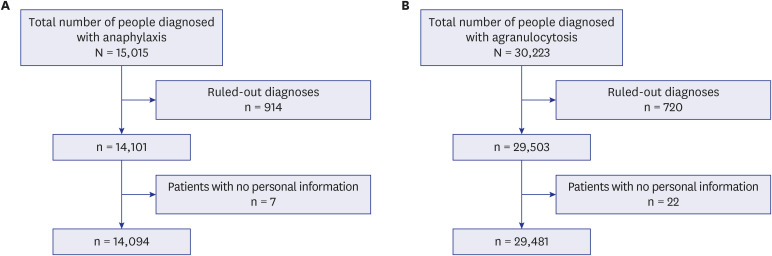
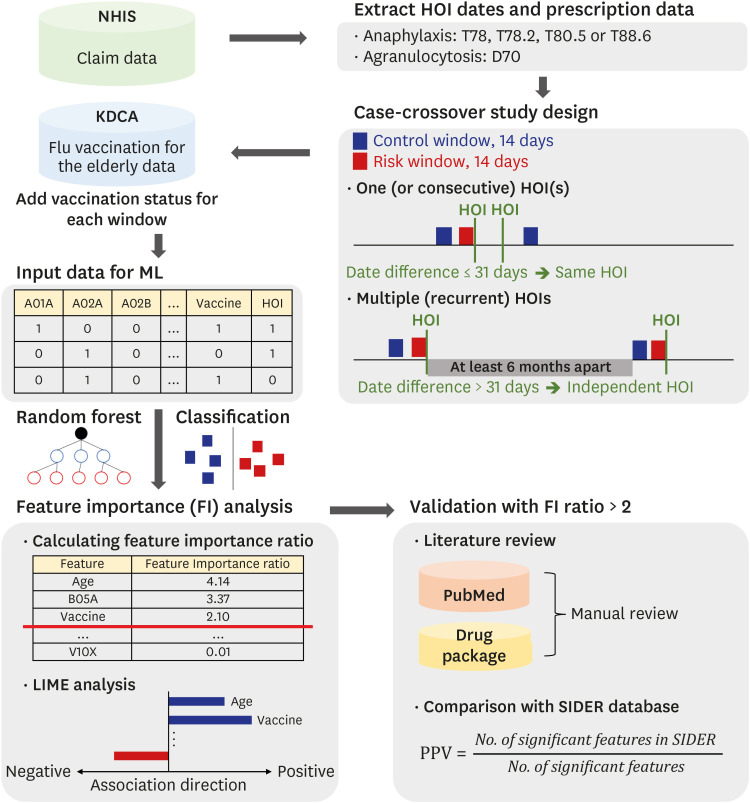
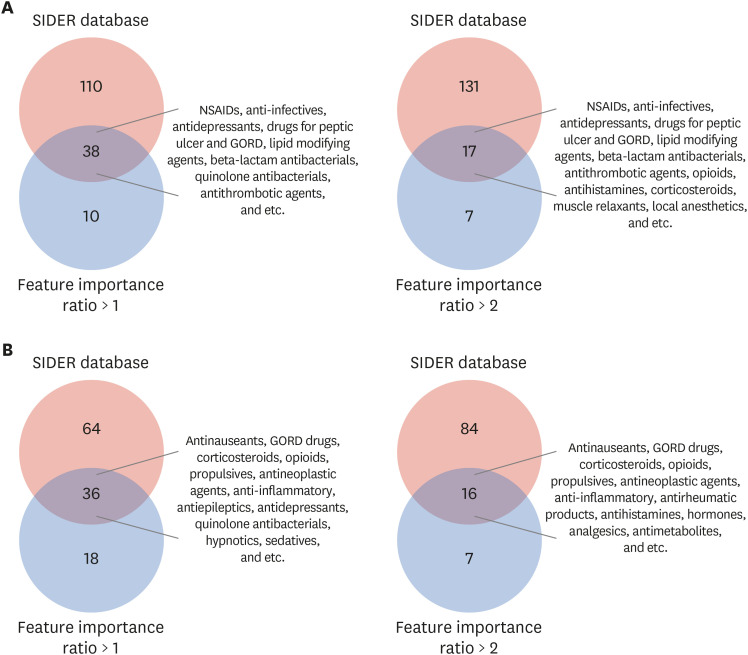
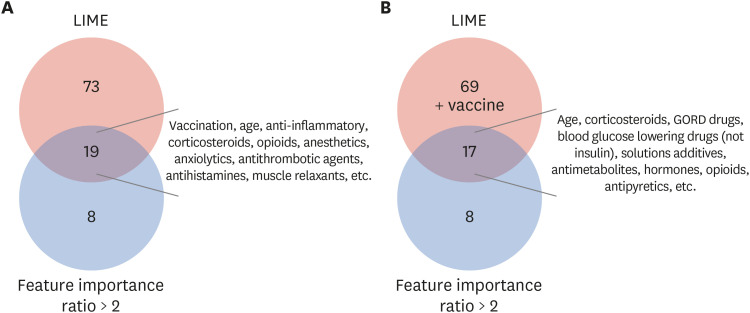
We used two databases, one from the Korea Disease Control and Prevention Agency, which contains flu vaccination records for the elderly, and another from the National Health Insurance Service, which contains the claim data of vaccinated people. We developed a casecrossover design based machine learning model to predict the health outcome of interest events (anaphylaxis and agranulocytosis) using a random forest. Feature importance values were evaluated to determine candidate associations with each outcome. We investigated the relationship of the features to each event via a literature review, comparison with the Side Effect Resource, and using the Local Interpretable Model-agnostic Explanation method.
Results
The trained model predicted each health outcome of interest with a high accuracy (approximately 70%). We found literature supporting our results, and most of the important drug-related features were listed in the Side Effect Resource database as inducing the health outcome of interest. For anaphylaxis, flu vaccination ranked high in our feature importance analysis and had a positive association in Local Interpretable Model-Agnostic Explanation analysis. Although the feature importance of vaccination was lower for agranulocytosis, it also had a positive relationship in the Local Interpretable Model-Agnostic Explanation analysis.
Conclusion
We developed a machine learning-based active surveillance system for detecting possible factors that can induce adverse events using health claim and vaccination databases. The results of the study demonstrated a potentially useful application of two linked national health record databases. Our model can contribute to the establishment of a system for conducting active surveillance on vaccination.
Keyword
Figure
Reference
-
1. Rubin R. Difficult to determine herd immunity threshold for COVID-19. JAMA. 2020; 324(8):732.
Article2. Jung J. Preparing for the coronavirus disease (COVID-19) vaccination: evidence, plans, and implications. J Korean Med Sci. 2021; 36(7):e59. PMID: 33619920.
Article3. Salmon DA, Dudley MZ, Glanz JM, Omer SB. Vaccine hesitancy: causes, consequences, and a call to action. Vaccine. 2015; 33(Suppl 4):D66–71. PMID: 26615171.4. Loomba S, de Figueiredo A, Piatek SJ, de Graaf K, Larson HJ. Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA. Nat Hum Behav. 2021; 5(3):337–348. PMID: 33547453.
Article5. Dubé E, Laberge C, Guay M, Bramadat P, Roy R, Bettinger J. Vaccine hesitancy: an overview. Hum Vaccin Immunother. 2013; 9(8):1763–1773. PMID: 23584253.6. Takahashi H, Pool V, Tsai TF, Chen RT. The VAERS Working Group. Adverse events after Japanese encephalitis vaccination: review of post-marketing surveillance data from Japan and the United States. Vaccine. 2000; 18(26):2963–2969. PMID: 10825597.
Article7. Jeong NY, Park S, Lim E, Choi NK. An introduction of the active vaccine safety surveillance system in foreign countries. J Health Info Stat. 2019; 44(4):317–330.
Article8. Choe YJ, Bae GR. Management of vaccine safety in Korea. Clin Exp Vaccine Res. 2013; 2(1):40–45. PMID: 23596589.
Article9. Davis RL, Kolczak M, Lewis E, Nordin J, Goodman M, Shay DK, et al. Active surveillance of vaccine safety: a system to detect early signs of adverse events. Epidemiology. 2005; 16(3):336–341. PMID: 15824549.10. Yih WK, Kulldorff M, Fireman BH, Shui IM, Lewis EM, Klein NP, et al. Active surveillance for adverse events: the experience of the Vaccine Safety Datalink project. Pediatrics. 2011; 127(Suppl 1):S54–64. PMID: 21502252.
Article11. Brown JS, Kulldorff M, Chan KA, Davis RL, Graham D, Pettus PT, et al. Early detection of adverse drug events within population-based health networks: application of sequential testing methods. Pharmacoepidemiol Drug Saf. 2007; 16(12):1275–1284. PMID: 17955500.
Article12. Huh K, Kim YE, Radnaabaatar M, Lee DH, Kim DW, Shin SA, et al. Estimating baseline incidence of conditions potentially associated with vaccine adverse events: a call for surveillance system using the Korean National Health Insurance Claims Data. J Korean Med Sci. 2021; 36(9):e67. PMID: 33686812.
Article13. Lee GM, Romero JR, Bell BP. Postapproval Vaccine Safety Surveillance for COVID-19 Vaccines in the US. JAMA. 2020; 324(19):1937–1938. PMID: 33064152.
Article14. Huang YL, Moon J, Segal JB. A comparison of active adverse event surveillance systems worldwide. Drug Saf. 2014; 37(8):581–596. PMID: 25022829.
Article15. Nikfarjam A, Sarker A, O’Connor K, Ginn R, Gonzalez G. Pharmacovigilance from social media: mining adverse drug reaction mentions using sequence labeling with word embedding cluster features. J Am Med Inform Assoc. 2015; 22(3):671–681. PMID: 25755127.
Article16. Botsis T, Nguyen MD, Woo EJ, Markatou M, Ball R. Text mining for the Vaccine Adverse Event Reporting System: medical text classification using informative feature selection. J Am Med Inform Assoc. 2011; 18(5):631–638. PMID: 21709163.
Article17. Jeon E, Kim Y, Park H, Park RW, Shin H, Park HA. Analysis of adverse drug reactions identified in nursing notes using reinforcement learning. Healthc Inform Res. 2020; 26(2):104–111. PMID: 32547807.
Article18. Lindquist M, Ståhl M, Bate A, Edwards IR, Meyboom RH. A retrospective evaluation of a data mining approach to aid finding new adverse drug reaction signals in the WHO international database. Drug Saf. 2000; 23(6):533–542. PMID: 11144660.
Article19. Tatonetti NP, Ye PP, Daneshjou R, Altman RB. Data-driven prediction of drug effects and interactions. Sci Transl Med. 2012; 4(125):125ra31.
Article20. Wang SV, Gagne JJ, Maro JC, Eworuke E, Kattinakere S, Kulldorff M, editors. Development and Evaluation of a Global Propensity Score for Data Mining with Tree-Based Scan Statistics. Sentinel;2018.21. Choi B, Kim SH, Lee H. Are registration of disease codes for adult anaphylaxis accurate in the emergency department? Allergy Asthma Immunol Res. 2018; 10(2):137–143. PMID: 29411554.
Article22. Helgeland J, Tomic O, Hansen TM, Kristoffersen DT, Hassani S, Lindahl AK. Postoperative wound dehiscence after laparotomy: a useful healthcare quality indicator? A cohort study based on Norwegian hospital administrative data. BMJ Open. 2019; 9(4):e026422.
Article23. Ribeiro MT, Singh S, Guestrin C. “Why should I trust you?” Explaining the predictions of any classifier. Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining. 2016. p. 1135–1144.
Article24. Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, et al. Scikit-learn: Machine learning in Python. Journal of machine Learning research. 2011; 12:2825–2830.25. Regateiro FS, Marques ML, Gomes ER. Drug-induced anaphylaxis: an update on epidemiology and risk factors. Int Arch Allergy Immunol. 2020; 181(7):481–487. PMID: 32396909.
Article26. Montañez MI, Mayorga C, Bogas G, Barrionuevo E, Fernandez-Santamaria R, Martin-Serrano A, et al. Epidemiology, mechanisms, and diagnosis of drug-induced anaphylaxis. Front Immunol. 2017; 8:614. PMID: 28611774.
Article27. Schweizer MT, Huang P, Kattan MW, Kibel AS, de Wit R, Sternberg CN, et al. Adjuvant leuprolide with or without docetaxel in patients with high-risk prostate cancer after radical prostatectomy (TAX-3501). Cancer. 2013; 119(20):3610–3618. PMID: 23943299.
Article28. Tse SS, Kish T. Octreotide-associated neutropenia. Pharmacotherapy. 2017; 37(6):e32–7. PMID: 28488730.
Article29. Kim MJ, Shim DH, Cha HR, Kim CB, Kim SY, Park JH, et al. Delayed-onset anaphylaxis caused by IgE response to influenza vaccination. Allergy Asthma Immunol Res. 2020; 12(2):359–363. PMID: 32009327.
Article30. Couronné R, Probst P, Boulesteix AL. Random forest versus logistic regression: a large-scale benchmark experiment. BMC Bioinformatics. 2018; 19(1):270. PMID: 30016950.
Article31. FDA. Ketorolac tromethamine. Updated 2014. Accessed March 3, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/074802s038lbl.pdf.32. Dhakal OP, Dhakal M, Bhandari D. Domperidone-induced dystonia: a rare and troublesome complication. BMJ Case Rep. 2014; 2014:bcr2013200282.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Application of Machine Learning in Rhinology: A State of the Art Review
- Novel Method of Classification in Knee Osteoarthritis: Machine Learning Application Versus Logistic Regression Model
- Applications of Machine Learning Using Electronic Medical Records in Spine Surgery
- Machine Learning vs. Statistical Model for Prediction Modelling: Application in Medical Imaging Research
- Artificial intelligence, machine learning, and deep learning in women’s health nursing