J Korean Med Sci.
2021 Aug;36(30):e218. 10.3346/jkms.2021.36.e218.
New-onset Nephrotic Syndrome after Janssen COVID-19 Vaccination: a Case Report and Literature Review
- Affiliations
-
- 1Division of Nephrology, Department of Internal Medicine, School of Medicine, Kyungpook National University, Kyungpook National University Hospital, Daegu, Korea
- 2Department of Pathology, School of Medicine, Kyungpook National University, Kyungpook National University Hospital, Daegu, Korea
- KMID: 2519198
- DOI: http://doi.org/10.3346/jkms.2021.36.e218
Abstract
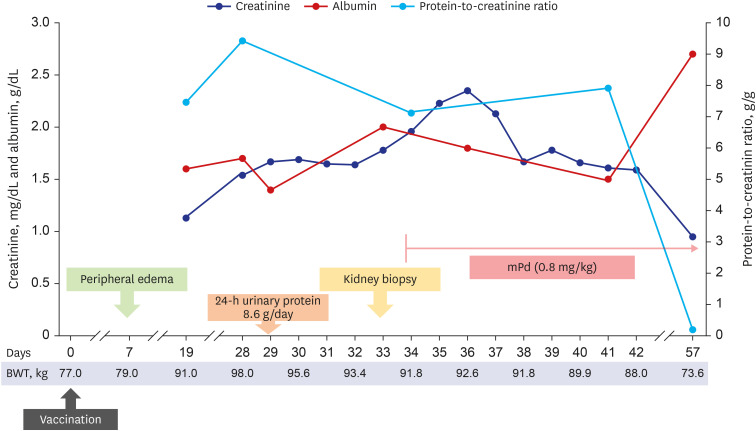
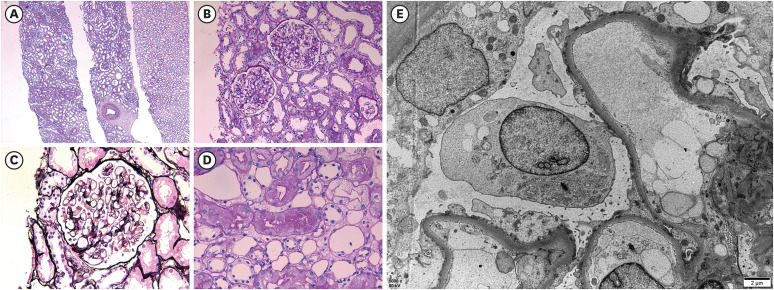
- Various coronavirus disease 2019 (COVID-19) vaccines are being developed, which show practical preventive effects. Here, we report a 51-year-old healthy man with nephrotic syndrome secondary to minimal change disease (MCD) after Ad26.COV.2 (Janssen) vaccination. He had no comorbid disease and received Ad26.COV.2 on April 13, 2021. Seven days after vaccination, he developed edema and foamy urine. Edema rapidly aggravated with decreased urine volume. He was admitted to the hospital 28 days after vaccination, and his body weight increased by 21 kg after vaccination. His serum creatinine level was 1.54 mg/ dL, and 24-h urinary protein excretion was 8.6 g/day. Kidney biopsy revealed no abnormality in the glomeruli and interstitium of the cortex and medulla under the light microscope. Electron microscopy revealed diffuse effacement of the podocyte foot processes, thus, he was diagnosed with MCD. High-dose steroid therapy was applied, and his kidney function improved three days after steroid therapy. Three weeks after steroid use, his serum creatinine decreased to 0.95 mg/dL, and spot urine protein-to-creatine decreased to 0.2 g/g. This case highlights the risk of new-onset nephrotic syndrome secondary to MCD after vectored COVID-19 vaccination. Although the pathogenesis is uncertain, clinicians need to be careful about adverse renal effects of COVID-19 vaccines.
Figure
Reference
-
1. U.S. Food & Drug Administration. Vaccines and Related Biological Products Advisory Committee Meeting. Updated 2021. Accessed May 24, 2021. https://www.fda.gov/advisory-committees/advisory-committee-calendar/vaccines-and-related-biological-products-advisory-committee-february-26-2021-meeting-announcement.2. Vivarelli M, Massella L, Ruggiero B, Emma F. Minimal change disease. Clin J Am Soc Nephrol. 2017; 12(2):332–345. PMID: 27940460.
Article3. Gupta RK, Bhargava R, Shaukat AA, Albert E, Leggat J. Spectrum of podocytopathies in new-onset nephrotic syndrome following COVID-19 disease: a report of 2 cases. BMC Nephrol. 2020; 21(1):326. PMID: 32753052.
Article4. Shah SA, Carter HP. New-onset nephrotic syndrome in a child associated with COVID-19 infection. Front Pediatr. 2020; 8:471. PMID: 32974243.
Article5. Lebedev L, Sapojnikov M, Wechsler A, Varadi-Levi R, Zamir D, Tobar A, et al. Minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis. 2021; 78(1):142–145. PMID: 33839200.
Article6. Maas RJ, Gianotten S, van der Meijden WA. An additional case of minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis. 2021; 78(2):312.
Article7. D'Agati VD, Kudose S, Bomback AS, Adamidis A, Tartini A. Minimal change disease and acute kidney injury following the Pfizer-BioNTech COVID-19 vaccine. Kidney Int. 2021; 100(2):461–463. PMID: 34000278.8. Holzworth A, Couchot P, Cruz-Knight W, Brucculeri M. Minimal change disease following the Moderna mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 2021; 100(2):463–464. PMID: 34048824.
Article9. Weijers J, Alvarez C, Hermans MM. Post-vaccinal minimal change disease. Kidney Int. 2021; 100(2):459–461.
Article10. Kervella D, Jacquemont L, Chapelet-Debout A, Deltombe C, Ville S. Minimal change disease relapse following SARS-CoV-2 mRNA vaccine. Kidney Int. 2021; 100(2):457–458. PMID: 33964312.
Article11. Morlidge C, El-Kateb S, Jeevaratnam P, Thompson B. Relapse of minimal change disease following the AstraZeneca COVID-19 vaccine. Kidney Int. 2021; 100(2):459.
Article12. Mancianti N, Guarnieri A, Tripodi S, Salvo DP, Garosi G. Minimal change disease following vaccination for SARS-CoV-2. J Nephrol. Forthcoming. 2021.
Article13. Komaba H, Wada T, Fukagawa M. Relapse of minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis. Forthcoming. 2021.
Article14. Schwotzer N, Kissling S, Fakhouri F. Letter regarding “Minimal change disease relapse following SARS-CoV-2 mRNA vaccine”. Kidney Int. 2021; 100(2):458–459. PMID: 34052236.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Early-Onset Myasthenia Gravis Following COVID-19 Vaccination
- Kidney complications associated with COVID-19 infection and vaccination in children and adolescents: a brief review
- Impact of COVID-19 on the clinical course of nephrotic syndrome in children: a single-center study
- Adult-Onset Still’s Disease after COVID-19 Vaccination: A Case Report and Review
- Alopecia areata after COVID-19 vaccination