Korean J Orthod.
2021 Jul;51(4):282-292. 10.4041/kjod.2021.51.4.282.
Effect of nicotine on orthodontic tooth movement and bone remodeling in rats
- Affiliations
-
- 1Department of Orthodontics, College of Dentistry, Yonsei University, Seoul, Korea
- 2Department of Orthodontics, Institute of Craniofacial Deformity, College of Dentistry, Yonsei University, Seoul, Korea
- 3Department of Preventive Dentistry of Public Oral Health, College of Dentistry, Yonsei University, Seoul, Korea
- 4Department of Periodontics, College of Dentistry, Yonsei University, Seoul, Korea
- KMID: 2518521
- DOI: http://doi.org/10.4041/kjod.2021.51.4.282
Abstract
Objective
To quantitatively analyze the effect of nicotine on orthodontic tooth movement (OTM) and bone remodeling in rats using micro-computed tomography and tartrate-resistant acid phosphatase immunostaining.
Methods
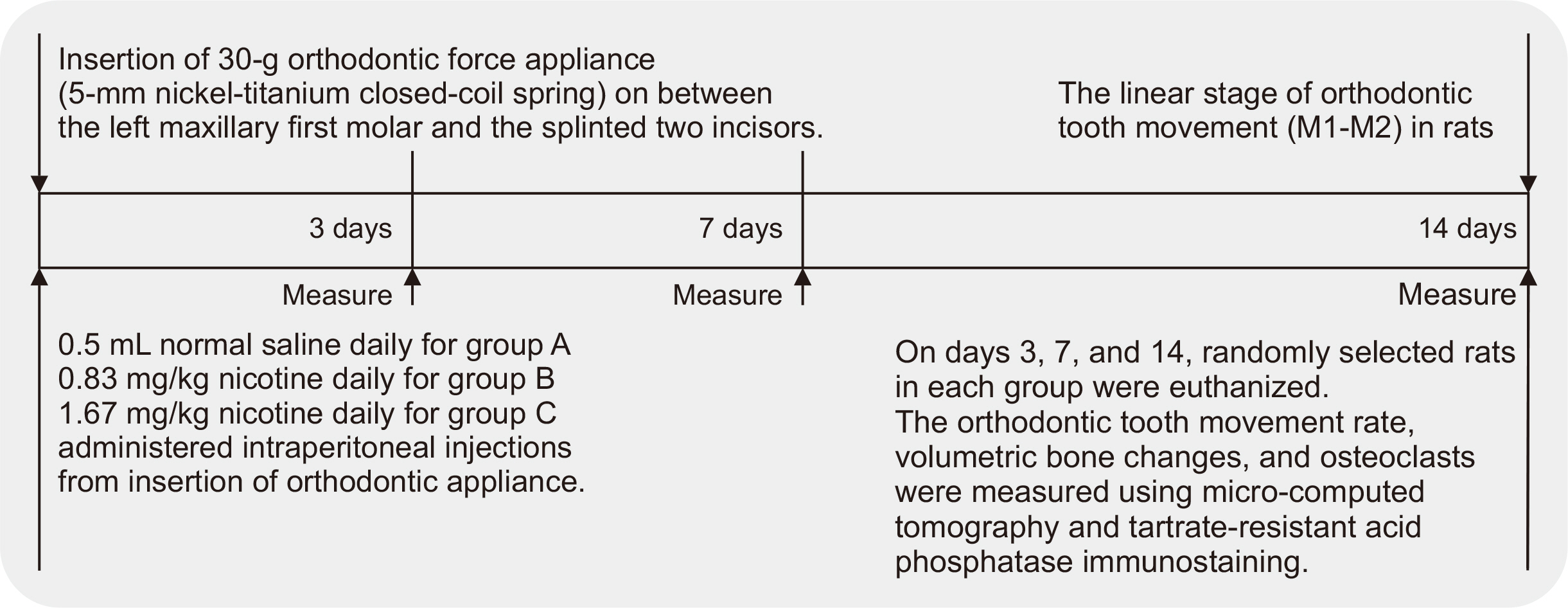
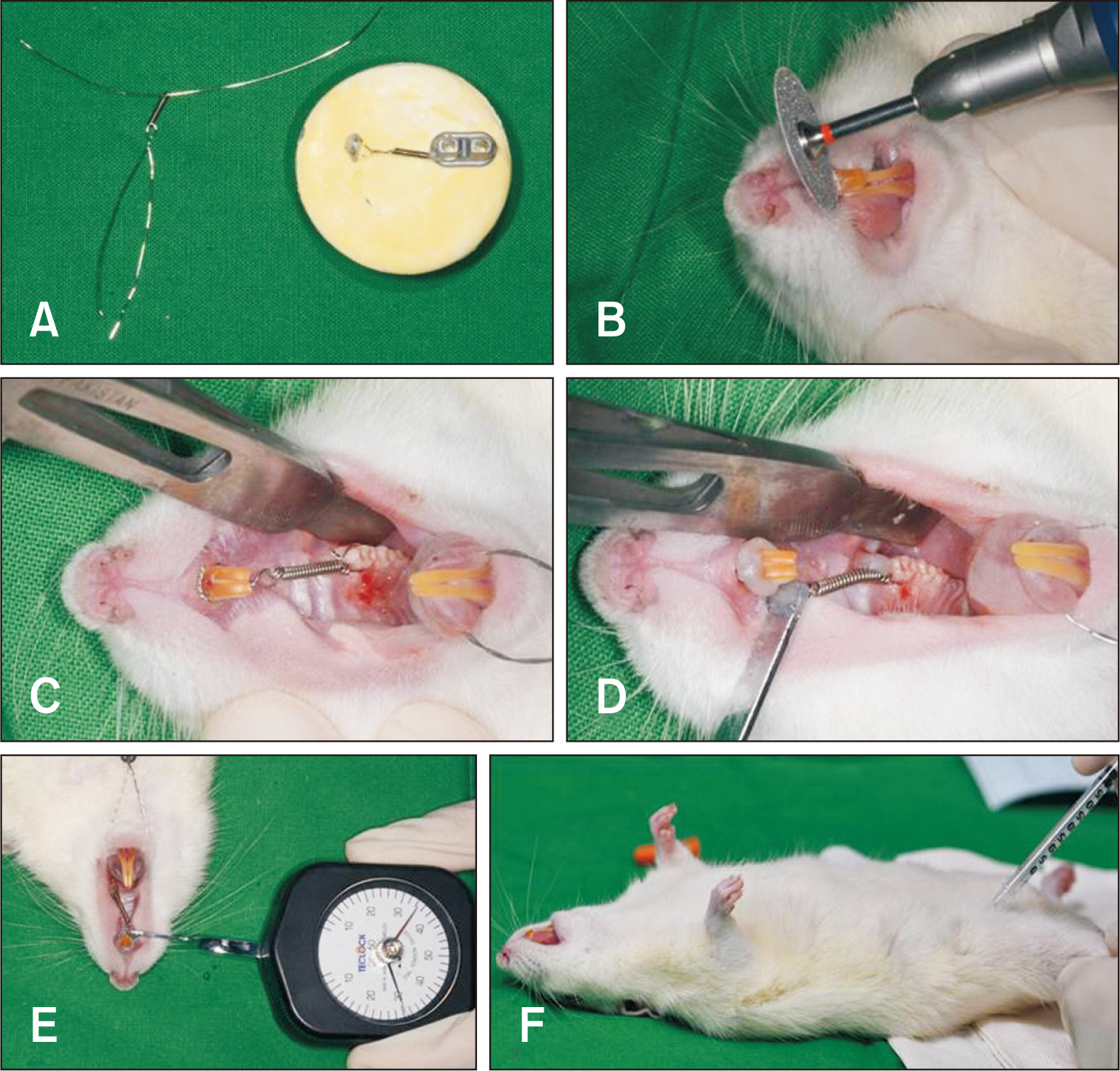
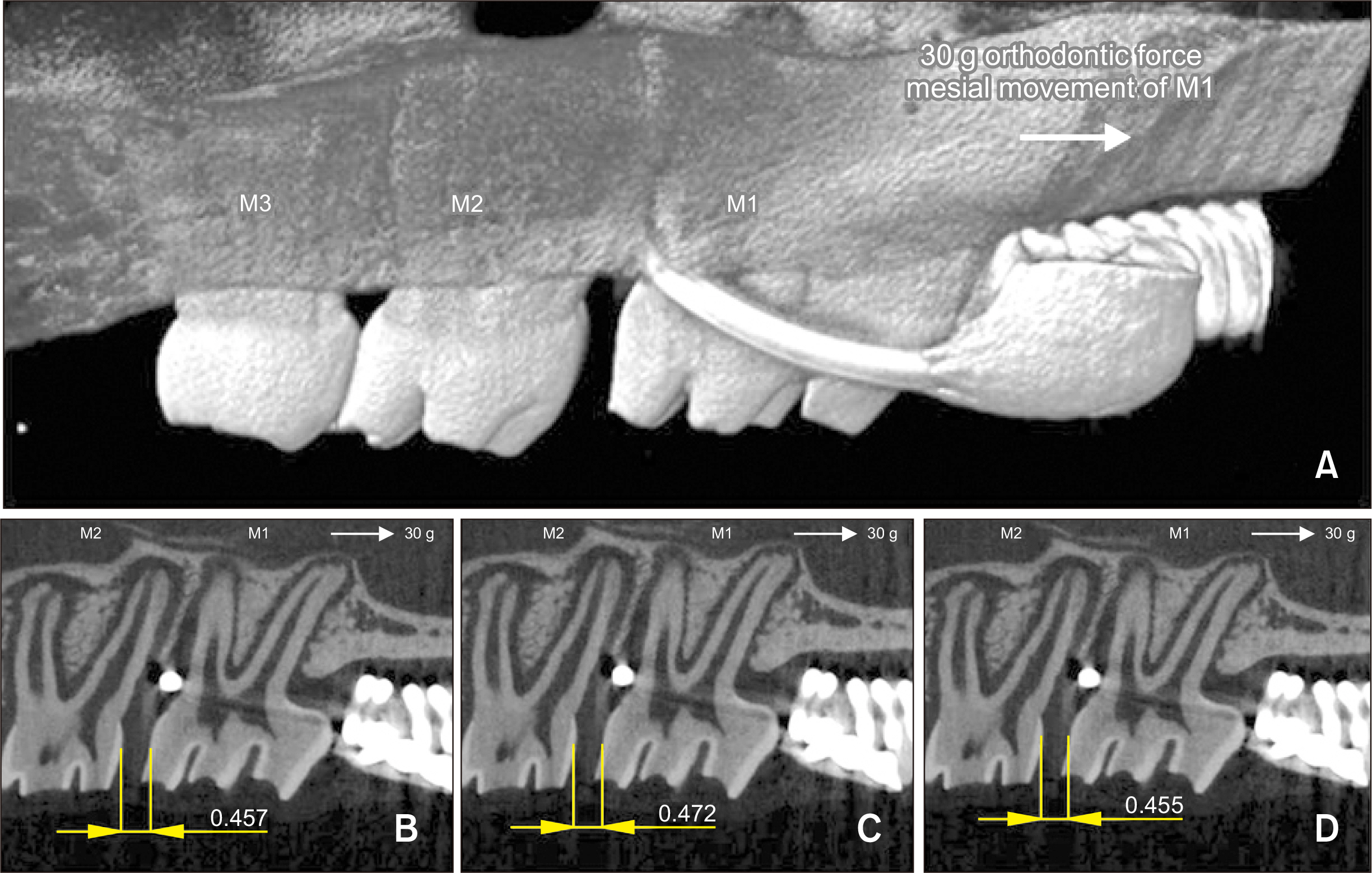
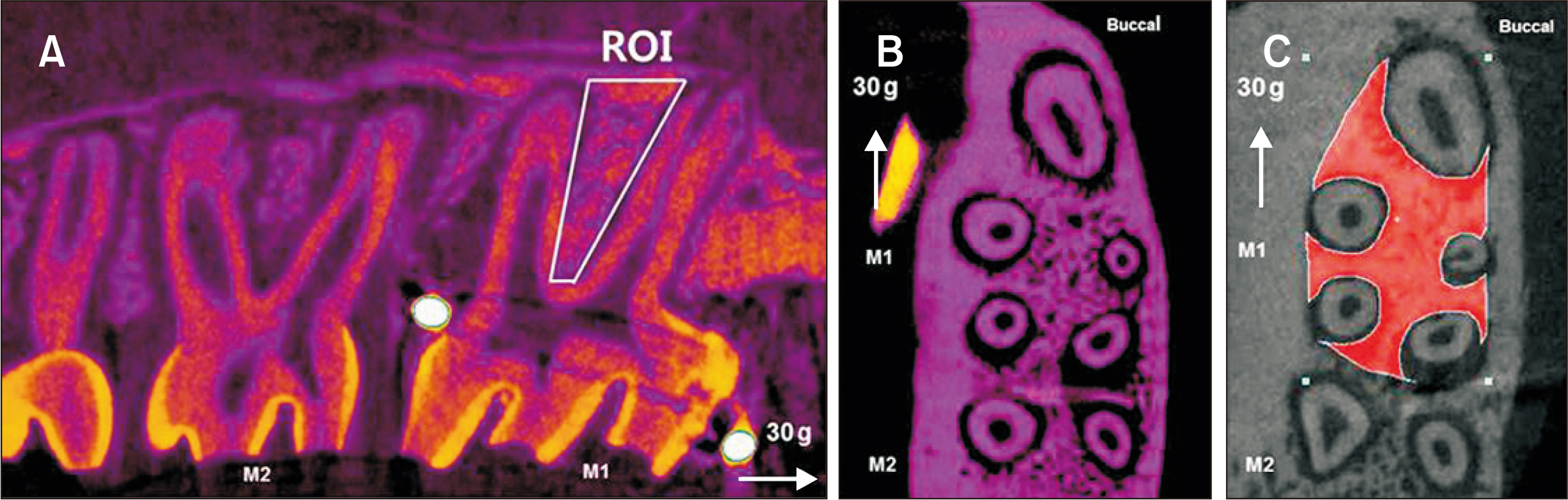
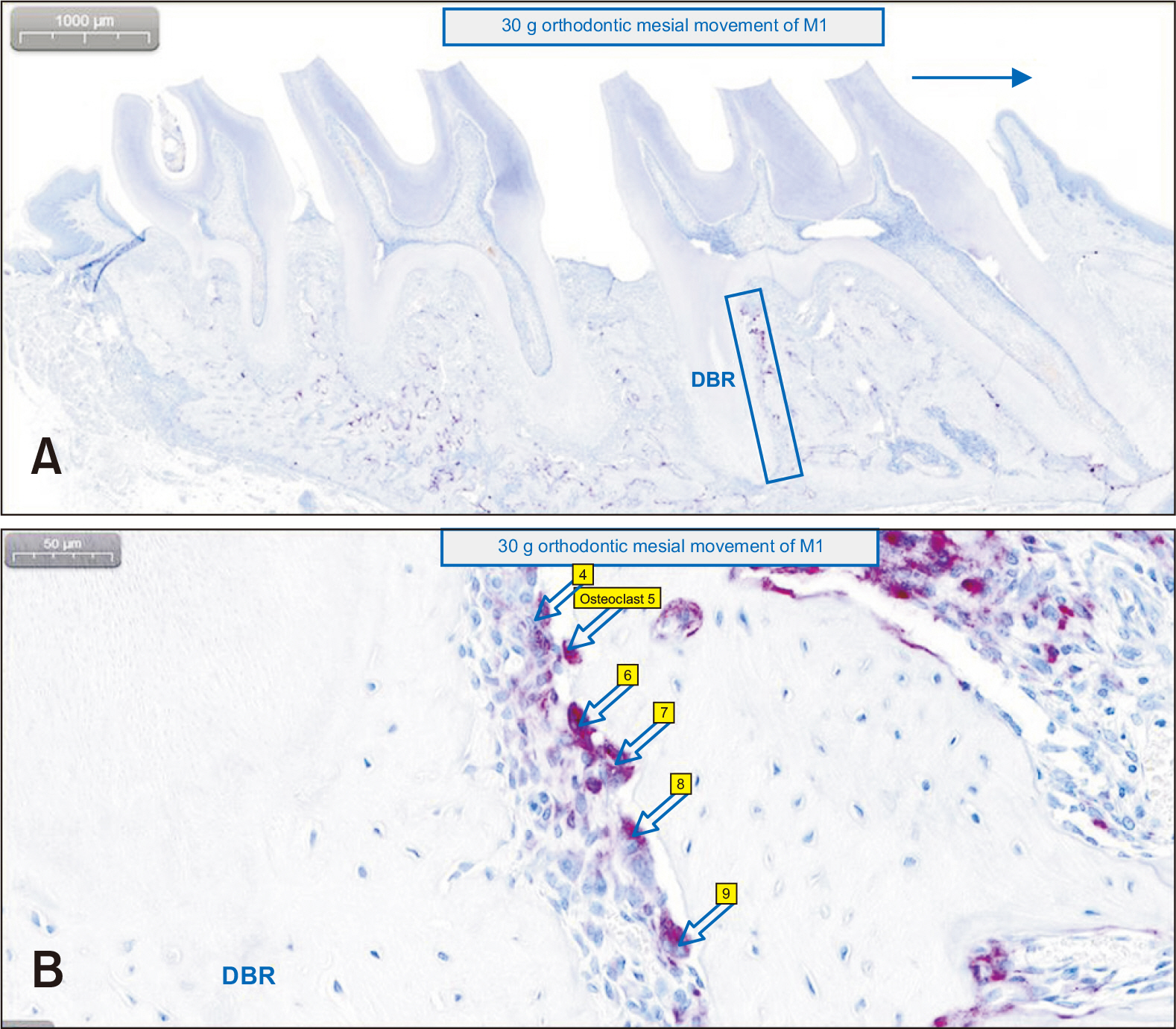
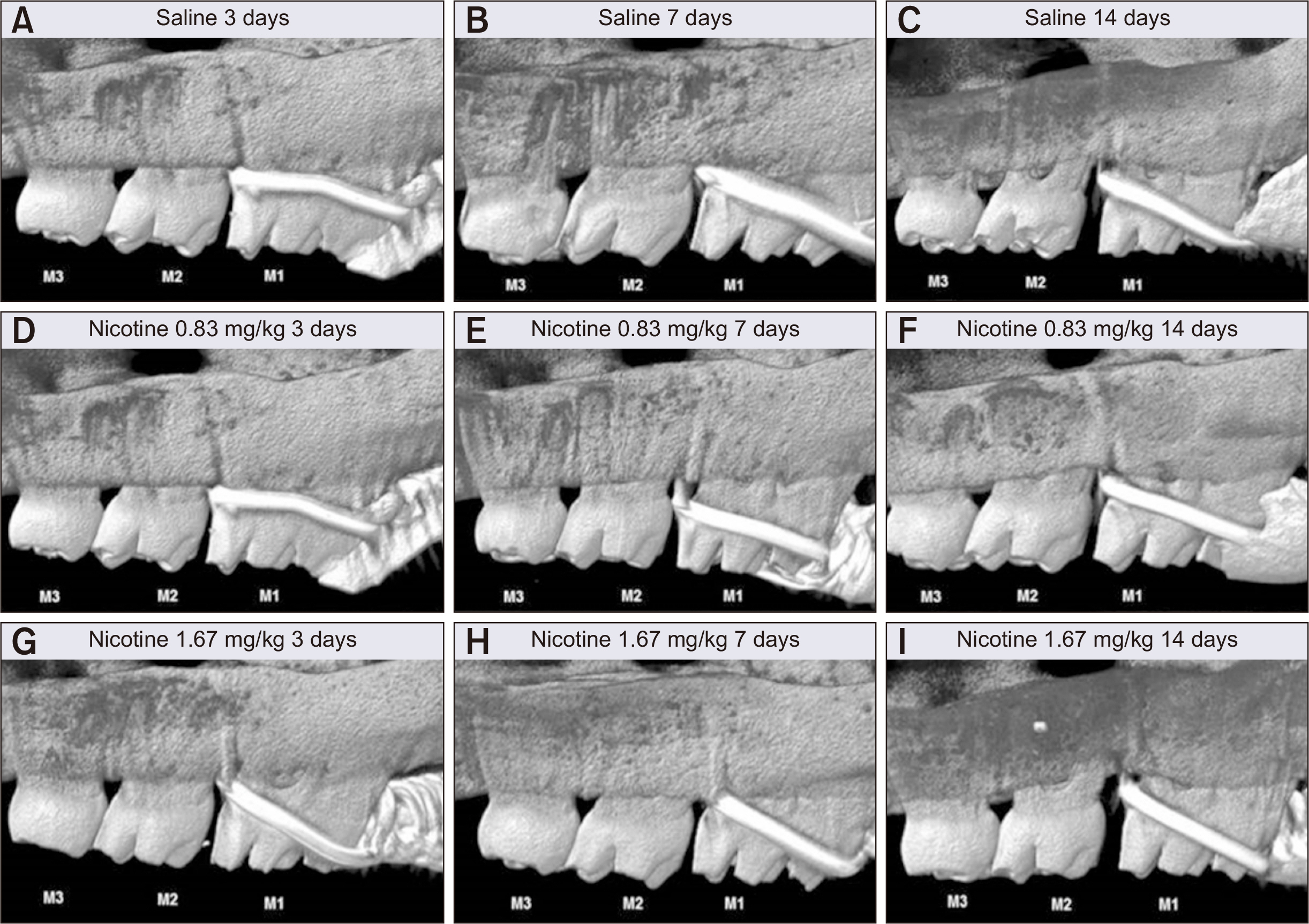
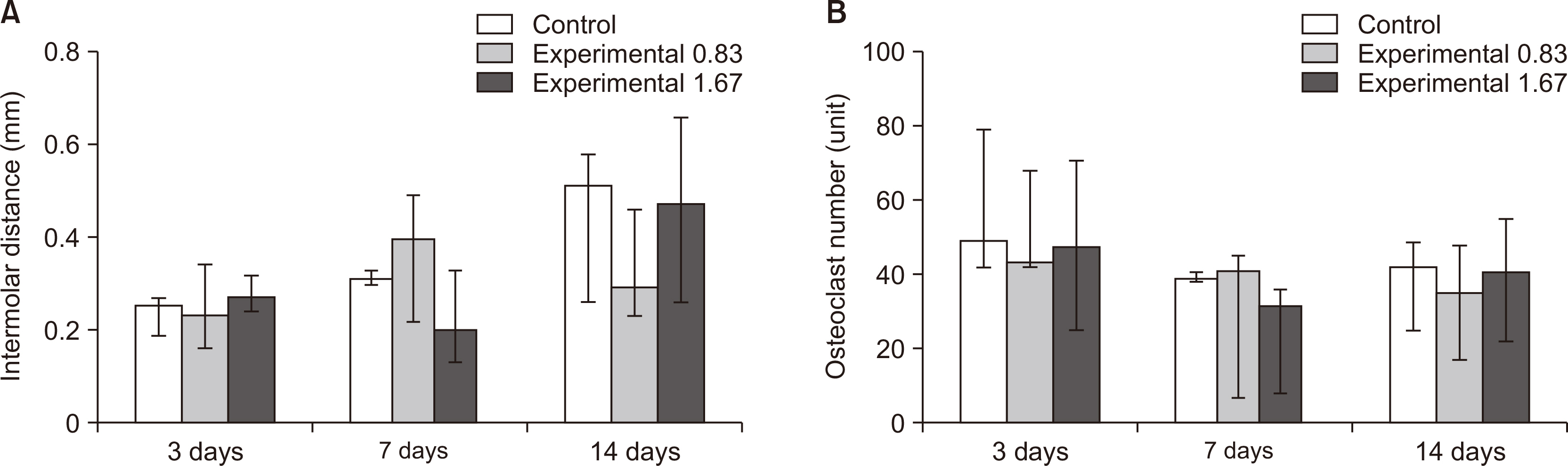
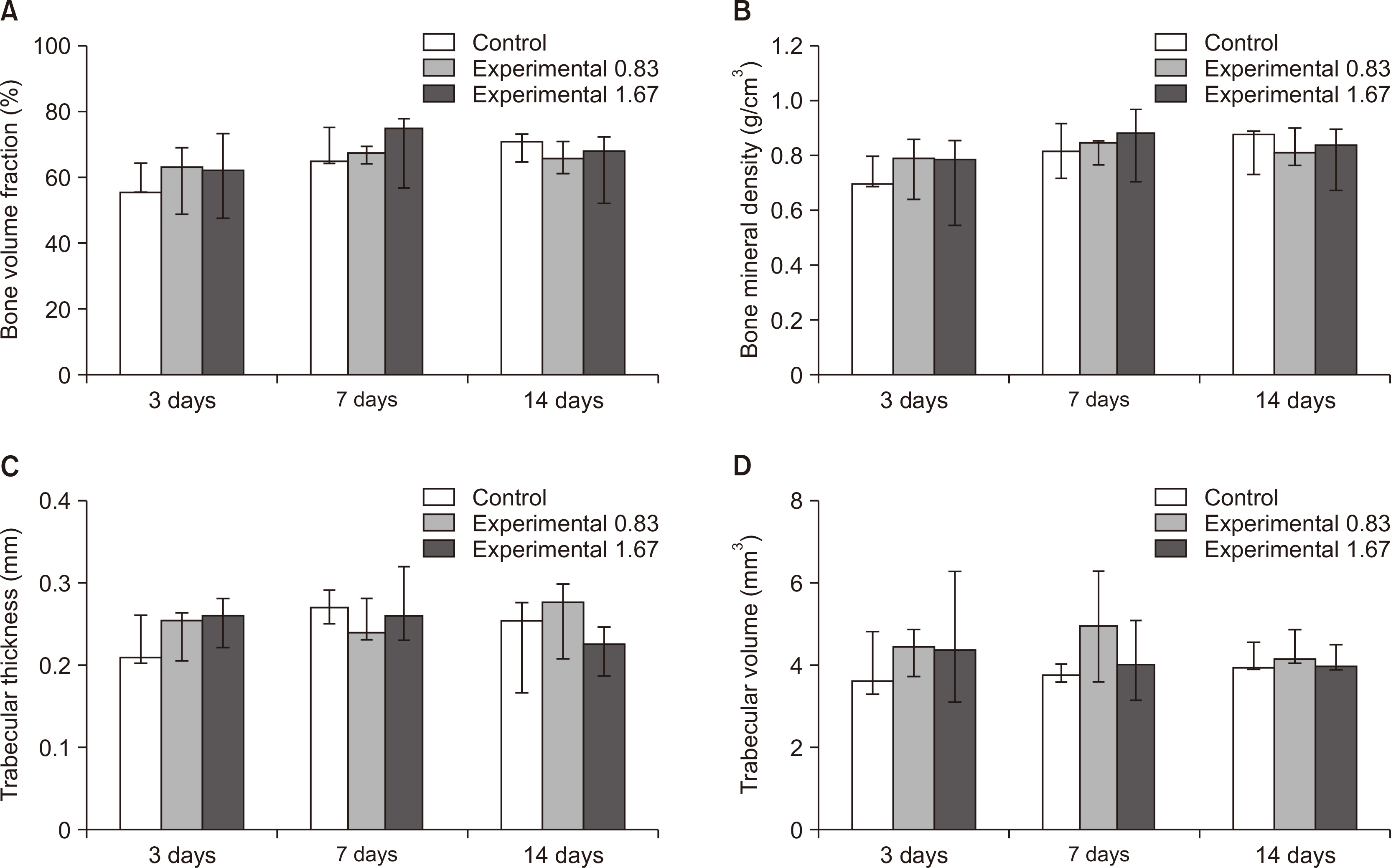
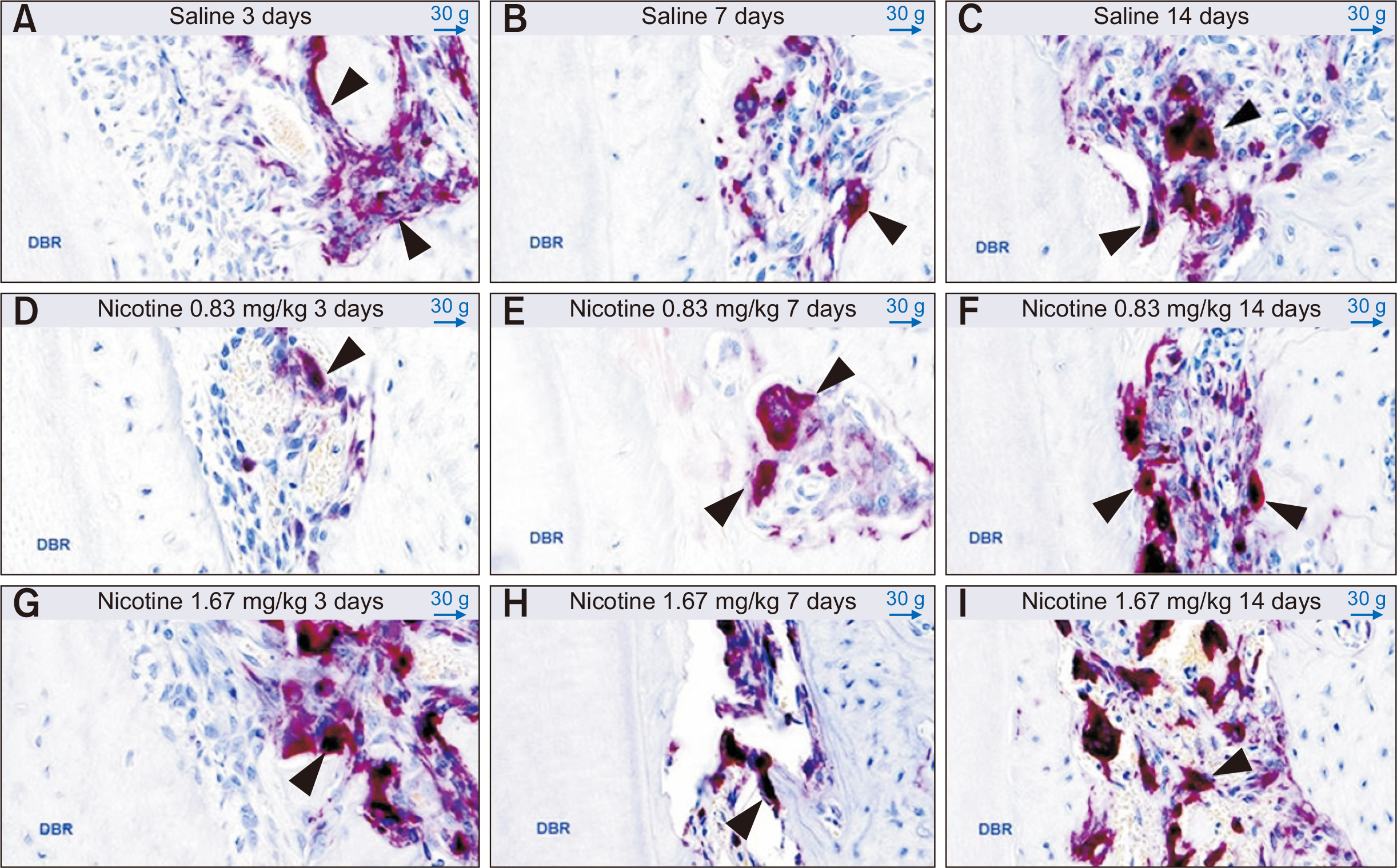
Thirty-nine adult male Sprague–Dawley rats were randomized into three groups: group A, 0.5 mL normal saline (n = 9, 3 per 3, 7, and 14 days); group B, 0.83 mg/kg nicotine (n = 15, 5 per 3, 7, and 14 days); and group C, 1.67 mg/kg nicotine (n = 15, 5 per 3, 7, and 14 days). Each animal received daily intraperitoneal injections of nicotine/saline from the day of insertion of identical 30-g orthodontic force delivery systems. A 5-mm nickel-titanium closed-coil spring was applied between the left maxillary first molar (M1) and the two splinted incisors. The rate of OTM and volumetric bone changes were measured using micro-computed tomography. Osteoclasts were counted on the mesial alveolar bone surface of the distobuccal root of M1. Six dependent outcome variables, including the intermolar distance, bone volume fraction, bone mineral density, trabecular thickness, trabecular volume, and osteoclast number, were summarized using simple descriptive statistics. Nonparametric Kruskal–Wallis tests were used to evaluate differences among groups at 3, 7, and 14 days of OTM.
Results
All six dependent outcome variables showed no statistically significant among group-differences at 3, 7, and 14 days.
Conclusions
The findings of this study suggest that nicotine does not affect OTM and bone remodeling, although fluctuations during the different stages of OTM in the nicotine groups should be elucidated in further prospective studies.
Figure
Reference
-
1. César-Neto JB, Duarte PM, Sallum EA, Barbieri D, Moreno H Jr, Nociti FH Jr. 2003; A comparative study on the effect of nicotine administration and cigarette smoke inhalation on bone healing around titanium implants. J Periodontol. 74:1454–9. DOI: 10.1902/jop.2003.74.10.1454. PMID: 14653391.
Article2. Hapidin H, Othman F, Soelaiman IN, Shuid AN, Luke DA, Mohamed N. 2007; Negative effects of nicotine on bone-resorbing cytokines and bone histomorphometric parameters in male rats. J Bone Miner Metab. 25:93–8. DOI: 10.1007/s00774-006-0733-9. PMID: 17323178.
Article3. Suda N, Morita I, Kuroda T, Murota S. 1993; Participation of oxidative stress in the process of osteoclast differentiation. Biochim Biophys Acta. 1157:318–23. DOI: 10.1016/0304-4165(93)90116-P. PMID: 8323962.
Article4. Mody N, Parhami F, Sarafian TA, Demer LL. 2001; Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med. 31:509–19. DOI: 10.1016/S0891-5849(01)00610-4. PMID: 11498284.
Article5. Bosco AF, Bonfante S, de Almeida JM, Luize DS, Nagata MJ, Garcia VG. 2007; A histologic and histometric assessment of the influence of nicotine on alveolar bone loss in rats. J Periodontol. 78:527–32. DOI: 10.1902/jop.2007.060149. PMID: 17335377.
Article6. Liu YF, Wu LA, Wang J, Wen LY, Wang XJ. 2010; Micro-computerized tomography analysis of alveolar bone loss in ligature- and nicotine-induced experimental periodontitis in rats. J Periodontal Res. 45:714–9. DOI: 10.1111/j.1600-0765.2010.01290.x. PMID: 20572916.
Article7. César-Neto JB, Benatti BB, Sallum EA, Casati MZ, Nociti FH Jr. 2006; The influence of cigarette smoke inhalation and its cessation on the tooth-supporting alveolar bone: a histometric study in rats. J Periodontal Res. 41:118–23. DOI: 10.1111/j.1600-0765.2005.00844.x. PMID: 16499714.
Article8. Krishnan V, Davidovitch Z. 2006; Cellular, molecular, and tissue-level reactions to orthodontic force. Am J Orthod Dentofacial Orthop. 129:469.e1–32. DOI: 10.1016/j.ajodo.2005.10.007. PMID: 16627171.
Article9. Gameiro GH, Pereira-Neto JS, Magnani MB, Nouer DF. 2007; The influence of drugs and systemic factors on orthodontic tooth movement. J Clin Orthod. 41:73–8. quiz 71PMID: 17473406.10. Iino S, Sakoda S, Ito G, Nishimori T, Ikeda T, Miyawaki S. 2007; Acceleration of orthodontic tooth movement by alveolar corticotomy in the dog. Am J Orthod Dentofacial Orthop. 131:448.e1–8. DOI: 10.1016/j.ajodo.2006.08.014. PMID: 17418709.
Article11. Yadav S, Dobie T, Assefnia A, Gupta H, Kalajzic Z, Nanda R. 2015; Effect of low-frequency mechanical vibration on orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 148:440–9. DOI: 10.1016/j.ajodo.2015.03.031. PMID: 26321342.12. Sodagar A, Donyavi Z, Arab S, Kharrazifard MJ. 2011; Effect of nicotine on orthodontic tooth movement in rats. Am J Orthod Dentofacial Orthop. 139:e261–5. DOI: 10.1016/j.ajodo.2010.08.018. PMID: 21392670.
Article13. Bakathir MA, Linjawi AI, Omar SS, Aboqura AB, Hassan AH. 2016; Effects of nicotine on bone during orthodontic tooth movement in male rats. Histological and immunohistochemical study. Saudi Med J. 37:1127–35. DOI: 10.15537/smj.2016.10.15159. PMID: 27652365. PMCID: PMC5075378.14. Shintcovsk RL, Knop L, Tanaka OM, Maruo H. 2014; Nicotine effect on bone remodeling during orthodontic tooth movement: histological study in rats. Dental Press J Orthod. 19:96–107. DOI: 10.1590/2176-9451.19.2.096-107.oar. PMID: 24945520. PMCID: PMC4296601.
Article15. Eckelman WC, Kilbourn MR, Joyal JL, Labiris R, Valliant JF. 2007; Justifying the number of animals for each experiment. Nucl Med Biol. 34:229–32. DOI: 10.1016/j.nucmedbio.2007.01.005. PMID: 17383571.
Article16. Scheibe PO. 2008; Number of samples - hypothesis testing. Nucl Med Biol. 35:3–9. DOI: 10.1016/j.nucmedbio.2007.10.006. PMID: 18158937.
Article17. Nociti FH Jr, Nogueira-Filho GR, Tramontina VA, Machado MA, Barros SP, Sallum EA, et al. 2001; Histometric evaluation of the effect of nicotine administration on periodontal breakdown: an in vivo study. J Periodontal Res. 36:361–6. DOI: 10.1034/j.1600-0765.2001.360603.x. PMID: 11762871.
Article18. Gonzales C, Hotokezaka H, Yoshimatsu M, Yozgatian JH, Darendeliler MA, Yoshida N. 2008; Force magnitude and duration effects on amount of tooth movement and root resorption in the rat molar. Angle Orthod. 78:502–9. DOI: 10.2319/052007-240.1. PMID: 18416627.
Article19. Reitan K, Kvam E. 1971; Comparative behavior of human and animal tissue during experimental tooth movement. Angle Orthod. 41:1–14. DOI: 10.1043/0003-3219(1971)041<0001:CBOHAA>2.0.CO;2. PMID: 4992550.20. Brudvik P, Rygh P. 1993; The initial phase of orthodontic root resorption incident to local compression of the periodontal ligament. Eur J Orthod. 15:249–63. DOI: 10.1093/ejo/15.4.249. PMID: 7691628.
Article21. Ren Y, Maltha JC, Kuijpers-Jagtman AM. 2004; The rat as a model for orthodontic tooth movement--a critical review and a proposed solution. Eur J Orthod. 26:483–90. DOI: 10.1093/ejo/26.5.483. PMID: 15536836.
Article22. Michelogiannakis D, Rossouw PE, Al-Shammery D, Akram Z, Khan J, Romanos GE, et al. 2018; Influence of nicotine on orthodontic tooth movement: a systematic review of experimental studies in rats. Arch Oral Biol. 93:66–73. DOI: 10.1016/j.archoralbio.2018.05.016. PMID: 29843070.
Article23. King GJ, Keeling SD, McCoy EA, Ward TH. 1991; Measuring dental drift and orthodontic tooth movement in response to various initial forces in adult rats. Am J Orthod Dentofacial Orthop. 99:456–65. DOI: 10.1016/S0889-5406(05)81579-3. PMID: 2028935.
Article24. Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R. 2010; Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 25:1468–86. DOI: 10.1002/jbmr.141. PMID: 20533309.
Article25. Walker LM, Preston MR, Magnay JL, Thomas PB, El Haj AJ. 2001; Nicotinic regulation of c-fos and osteopontin expression in human-derived osteoblast-like cells and human trabecular bone organ culture. Bone. 28:603–8. DOI: 10.1016/S8756-3282(01)00427-6. PMID: 11425648.
Article26. Henemyre CL, Scales DK, Hokett SD, Cuenin MF, Peacock ME, Parker MH, et al. 2003; Nicotine stimulates osteoclast resorption in a porcine marrow cell model. J Periodontol. 74:1440–6. DOI: 10.1902/jop.2003.74.10.1440. PMID: 14653389.
Article27. Kirschneck C, Proff P, Maurer M, Reicheneder C, Römer P. 2015; Orthodontic forces add to nicotine-induced loss of periodontal bone: an in vivo and in vitro study. J Orofac Orthop. 76:195–212. DOI: 10.1007/s00056-015-0283-7. PMID: 25929709.28. Araujo CM, Rocha AC, Araujo BMM, Johann ACBR, Pereira LF, Tanaka OM, et al. 2018; Effect of acute administration of nicotine and ethanol on tooth movement in rats. Braz Oral Res. 32:e96. DOI: 10.1590/1807-3107bor-2018.vol32.0096. PMID: 30328897.
Article29. Lee HJ, Pi SH, Kim Y, Kim HS, Kim SJ, Kim YS, et al. 2009; Effects of nicotine on antioxidant defense enzymes and RANKL expression in human periodontal ligament cells. J Periodontol. 80:1281–8. DOI: 10.1902/jop.2009.090098. PMID: 19656028.
Article30. Norazlina M, Hermizi H, Faizah O, Nazrun AS, Norliza M, Ima-Nirwana S. 2010; Vitamin E reversed nicotine-induced toxic effects on bone biochemical markers in male rats. Arch Med Sci. 6:505–12. DOI: 10.5114/aoms.2010.14460. PMID: 22371792. PMCID: PMC3284063.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of vitamin C deficiency on the rate of orthodontic tooth movement and alveolar bone remodeling
- The limitation of alveolar bone remodeling during retraction of the upper anterior teeth
- A study on cyclic AMP in alveolar bone treated by orthodontic forces
- A study on the effects of Electrical stimulation by the miniature Electric device on the tooth movement and tissue remodeling
- Interleukin-1beta levels in human gingival crevicular fluid during orthodontic tooth movement