Cancer Res Treat.
2021 Jul;53(3):803-812. 10.4143/crt.2020.1251.
Early Metabolic Response Assessed Using 18F-FDG-PET/CT for Image-Guided Intracavitary Brachytherapy Can Better Predict Treatment Outcomes in Patients with Cervical Cancer
- Affiliations
-
- 1Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Department of Obstetrics and Gynecology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 3Department of Nuclear Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2518404
- DOI: http://doi.org/10.4143/crt.2020.1251
Abstract
- Purpose
This study aimed to identify the prognostic value of early metabolic response assessed using 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) during radiation therapy (RT) for cervical cancer.
Materials and Methods
We identified 116 patients treated with definitive RT, including FDG-PET/CT–guided intracavitary brachytherapy, between 2009 and 2018. We calculated parameters including maximum (SUVmax) and mean standardized uptake values (SUVmean), metabolic tumor volume (MTV), and total lesion glycolysis (TLG) for baseline FDG-PET/CT (PETbase) and image-guided brachytherapy planning FDG-PET/CT (PETIGBT). Multivariable analyses of disease-free survival (DFS) and overall survival (OS) were performed.
Results
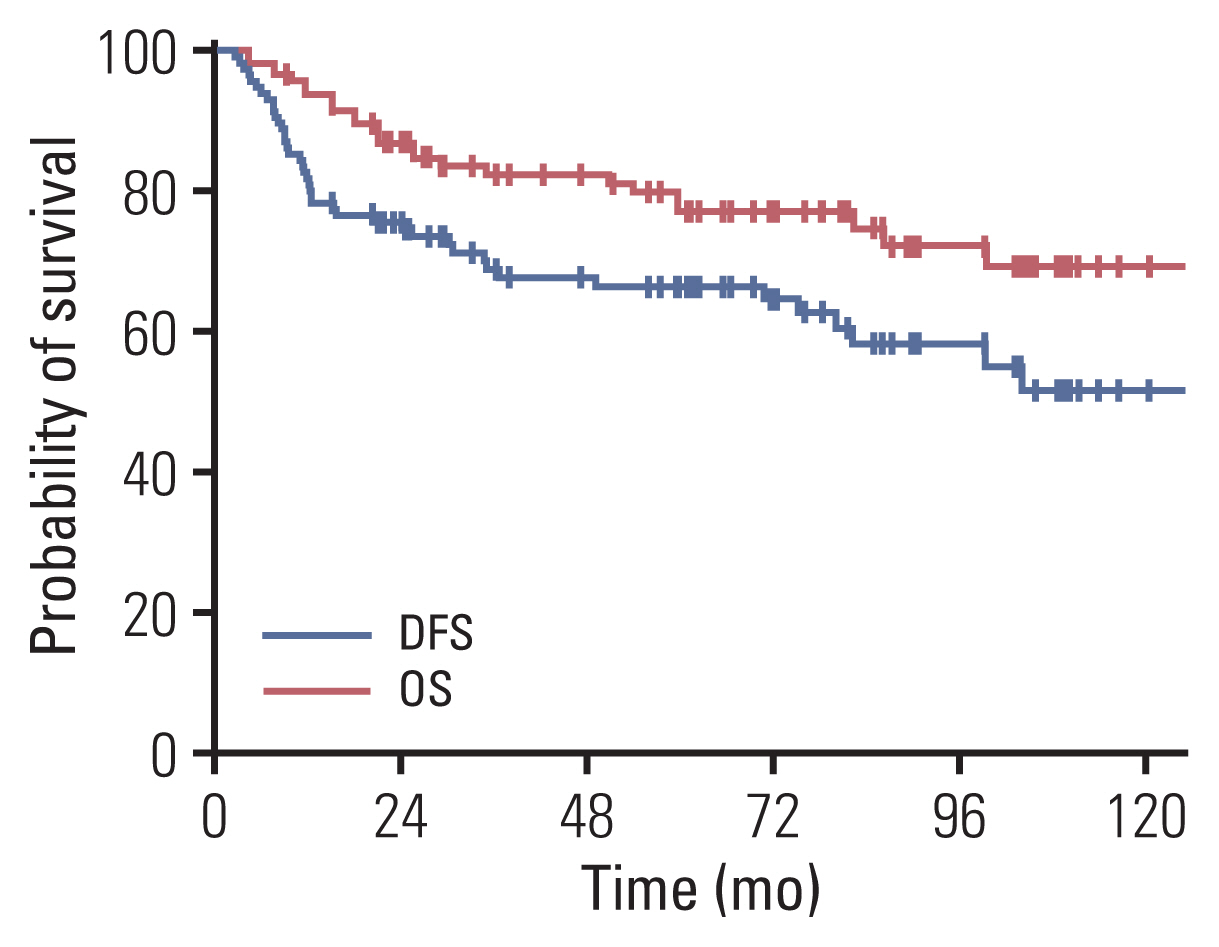
We observed a time-dependent decrease in PET parameters between PETbase and PETIGBT; ΔSUVmax, ΔSUVmean, ΔMTV, and ΔTLG were 65%, 61%, 78%, and 93%, respectively. With a median follow-up of 59.5 months, the 5-year DFS and OS rates were 66% and 79%, respectively. Multivariable analysis demonstrated that ΔSUVmax ≥ 50% was associated with favorable DFS (hazard ratio [HR], 2.56; 95% confidence interval [CI], 1.14 to 5.77) and OS (HR, 5.14; 95% CI, 1.55 to 17.01). Patients with ΔSUVmax ≥ 50% (n=87) showed better DFS and OS than those with ΔSUVmax < 50% (n=29) (DFS, 76% vs. 35%, p < 0.001; OS, 90% vs. 41%, p < 0.001, respectively). Adenocarcinoma was frequently observed in ΔSUVmax < 50% compared to ΔSUVmax ≥ 50% (27.6% vs. 10.3%, p=0.003). In addition, models incorporating metabolic parameters showed improved accuracy for predicting DFS (p=0.012) and OS (p=0.004) than models with clinicopathologic factors.
Conclusion
Changes in metabolic parameters, especially those in SUVmax by > 50%, can help improve survival outcome predictions for patients with cervical cancer treated with definitive RT.
Keyword
Figure
Reference
-
References
1. Herrera FG, Prior JO. The role of PET/CT in cervical cancer. Front Oncol. 2013; 3:34.
Article2. Nam H, Huh SJ, Ju SG, Park W, Lee JE, Choi JY, et al. 18F-fluorodeoxyglucose positron emisson tomography/computed tomography guided conformal brachytherapy for cervical cancer. Int J Radiat Oncol Biol Phys. 2012; 84:e29–34.
Article3. Oh D, Huh SJ, Park W, Ju SG, Nam H, Lee JE. Clinical outcomes in cervical cancer patients treated by FDG-PET/CT-based 3-dimensional planning for the first brachytherapy session. Medicine (Baltimore). 2016; 95:e3895.
Article4. Engin G. Cervical cancer: MR imaging findings before, during, and after radiation therapy. Eur Radiol. 2006; 16:313–24.
Article5. Yoon JW, Kim S, Kim SW, Kim YT, Kang WJ, Nam EJ. PET/CT response criteria (European Organization for Research and Treatment of Cancer) predict survival better than response evaluation criteria in solid tumors in locally advanced cervical cancer treated with chemoradiation. Clin Nucl Med. 2016; 41:677–82.
Article6. Lordick F, Ott K, Krause BJ, Weber WA, Becker K, Stein HJ, et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol. 2007; 8:797–805.
Article7. Kim N, Cho H, Yun M, Park KR, Lee CG. Prognostic values of mid-radiotherapy (18)F-FDG PET/CT in patients with esophageal cancer. Radiat Oncol. 2019; 14:27.
Article8. Kim N, Kim JS, Geol Lee C. Predictive value of interim 18F-FDG-PET in patients with non-small cell lung cancer treated with definitive radiation therapy. PLoS One. 2020; 15:e0236350.
Article9. Kim S, Oh S, Kim JS, Kim YK, Kim KH, Oh DH, et al. Prognostic value of FDG PET/CT during radiotherapy in head and neck cancer patients. Radiat Oncol J. 2018; 36:95–102.
Article10. Haie-Meder C, Potter R, Van Limbergen E, Briot E, De Brabandere M, Dimopoulos J, et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (I): concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol. 2005; 74:235–45.
Article11. Kidd EA, Thomas M, Siegel BA, Dehdashti F, Grigsby PW. Changes in cervical cancer FDG uptake during chemoradiation and association with response. Int J Radiat Oncol Biol Phys. 2013; 85:116–22.
Article12. Lin LL, Yang Z, Mutic S, Miller TR, Grigsby PW. FDG-PET imaging for the assessment of physiologic volume response during radiotherapy in cervix cancer. Int J Radiat Oncol Biol Phys. 2006; 65:177–81.
Article13. Schwarz JK, Lin LL, Siegel BA, Miller TR, Grigsby PW. 18-F-Fluorodeoxyglucose-positron emission tomography evaluation of early metabolic response during radiation therapy for cervical cancer. Int J Radiat Oncol Biol Phys. 2008; 72:1502–7.
Article14. Leseur J, Roman-Jimenez G, Devillers A, Ospina-Arango JD, Williaume D, Castelli J, et al. Pre- and per-treatment 18F-FDG PET/CT parameters to predict recurrence and survival in cervical cancer. Radiother Oncol. 2016; 120:512–8.
Article15. Oh D, Lee JE, Huh SJ, Park W, Nam H, Choi JY, et al. Prognostic significance of tumor response as assessed by sequential 18F-fluorodeoxyglucose-positron emission tomography/computed tomography during concurrent chemoradiation therapy for cervical cancer. Int J Radiat Oncol Biol Phys. 2013; 87:549–54.
Article16. Shen WC, Chen SW, Wu KC, Hsieh TC, Liang JA, Hung YC, et al. Prediction of local relapse and distant metastasis in patients with definitive chemoradiotherapy-treated cervical cancer by deep learning from [(18)F]-fluorodeoxyglucose positron emission tomography/computed tomography. Eur Radiol. 2019; 29:6741–9.
Article17. Kidd EA, Siegel BA, Dehdashti F, Grigsby PW. The standardized uptake value for F-18 fluorodeoxyglucose is a sensitive predictive biomarker for cervical cancer treatment response and survival. Cancer. 2007; 110:1738–44.
Article18. Kidd EA, Siegel BA, Dehdashti F, Rader JS, Mutch DG, Powell MA, et al. Lymph node staging by positron emission tomography in cervical cancer: relationship to prognosis. J Clin Oncol. 2010; 28:2108–13.
Article19. Kidd EA, Siegel BA, Dehdashti F, Rader JS, Mutic S, Mutch DG, et al. Clinical outcomes of definitive intensity-modulated radiation therapy with fluorodeoxyglucose-positron emission tomography simulation in patients with locally advanced cervical cancer. Int J Radiat Oncol Biol Phys. 2010; 77:1085–91.
Article20. Nam H, Park W, Huh SJ, Bae DS, Kim BG, Lee JH, et al. The prognostic significance of tumor volume regression during radiotherapy and concurrent chemoradiotherapy for cervical cancer using MRI. Gynecol Oncol. 2007; 107:320–5.
Article21. Fields EC, Weiss E. A practical review of magnetic resonance imaging for the evaluation and management of cervical cancer. Radiat Oncol. 2016; 11:15.
Article22. Sun C, Tian X, Liu Z, Li W, Li P, Chen J, et al. Radiomic analysis for pretreatment prediction of response to neoadjuvant chemotherapy in locally advanced cervical cancer: a multicentre study. EBioMedicine. 2019; 46:160–9.
Article23. Wormald BW, Doran SJ, Ind TE, D’Arcy J, Petts J, deSouza NM. Radiomic features of cervical cancer on T2-and diffusion-weighted MRI: prognostic value in low-volume tumors suitable for trachelectomy. Gynecol Oncol. 2020; 156:107–14.
Article24. Lucia F, Visvikis D, Desseroit MC, Miranda O, Malhaire JP, Robin P, et al. Prediction of outcome using pretreatment (18)F-FDG PET/CT and MRI radiomics in locally advanced cervical cancer treated with chemoradiotherapy. Eur J Nucl Med Mol Imaging. 2018; 45:768–86.
Article25. Lucia F, Visvikis D, Vallieres M, Desseroit MC, Miranda O, Robin P, et al. External validation of a combined PET and MRI radiomics model for prediction of recurrence in cervical cancer patients treated with chemoradiotherapy. Eur J Nucl Med Mol Imaging. 2019; 46:864–77.
Article26. Lee JH, Lee SW, Kim JR, Kim YS, Yoon MS, Jeong S, et al. Tumour size, volume, and marker expression during radiation therapy can predict survival of cervical cancer patients: a multi-institutional retrospective analysis of KROG 16-01. Gynecol Oncol. 2017; 147:577–84.
Article27. Yang K, Park W, Huh SJ, Bae DS, Kim BG, Lee JW. Clinical outcomes in patients treated with radiotherapy after surgery for cervical cancer. Radiat Oncol J. 2017; 35:39–47.
Article28. Noh JM, Park W, Kim YS, Kim JY, Kim HJ, Kim J, et al. Comparison of clinical outcomes of adenocarcinoma and adenosquamous carcinoma in uterine cervical cancer patients receiving surgical resection followed by radiotherapy: a multicenter retrospective study (KROG 13-10). Gynecol Oncol. 2014; 132:618–23.
Article29. Hodgson A, Park KJ. Cervical adenocarcinomas: a heterogeneous group of tumors with variable etiologies and clinical outcomes. Arch Pathol Lab Med. 2019; 143:34–46.30. Hautzel H, Muller-Gartner HW. Early changes in fluorine-18-FDG uptake during radiotherapy. J Nucl Med. 1997; 38:1384–6.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Use of 18F-FDG PET/CT in Second Primary Cancer
- Early Assessment of Response to Neoadjuvant Chemotherapy with 18F-FDG-PET/CT in Patients with Advanced-Stage Ovarian Cancer
- Imaging of Gastric Cancer Metabolism Using 18 F-FDG PET/CT
- F-18 FDG PET/CT Findings of Subcutaneous Panniculitis - Like T- Cell Lymphoma : A Case Report
- PET Imaging for Gynecologic Malignancy