Cancer Res Treat.
2021 Jul;53(3):695-702. 10.4143/crt.2020.1246.
Implications of Tamoxifen Resistance in Palbociclib Efficacy for Patients with Hormone Receptor-Positive, HER2-Negative Metastatic Breast Cancer: Subgroup Analyses of KCSG-BR15-10 (YoungPEARL)
- Affiliations
-
- 1Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Department of Internal Medicine, Seoul National University Hospital, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea
- 3Division of Medical Oncology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea
- 4Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 5Department of Hematology-Oncology, Ajou University School of Medicine, Suwon, Korea
- 6Center for Breast Cancer, National Cancer Center, Goyang, Korea
- 7Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 8Division of Medical Oncology, Department of Internal Medicine, Gachon University Gil Medical Center, Incheon, Korea
- KMID: 2518393
- DOI: http://doi.org/10.4143/crt.2020.1246
Abstract
- Purpose
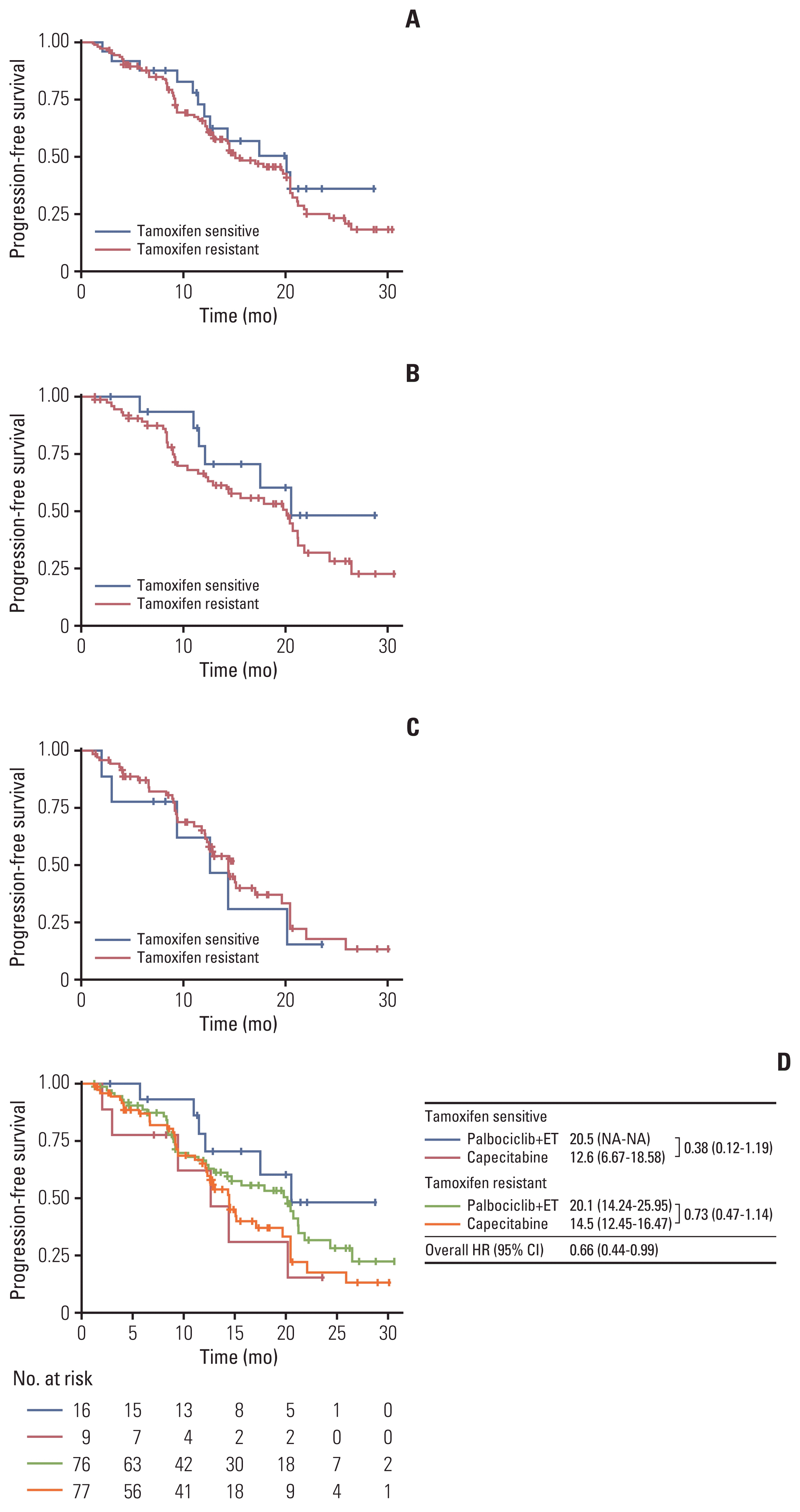
YoungPEARL (KCSG-BR15-10) trial demonstrated a significant progression-free survival (PFS) benefit for premenopausal patients with hormone receptor–positive/human epidermal growth factor receptor 2–negative (HR+/HER2–) metastatic breast cancer (MBC) for palbociclib plus exemestane with ovarian function suppression compared to capecitabine. However, the number of tamoxifen-sensitive premenopausal patients was small because most recurrences occurred early during adjuvant endocrine therapy (ET), with tamoxifen being the only drug used; hence, the data for these patients were limited. Here we present a subgroup analysis according to tamoxifen sensitivity from the YoungPEARL study. Materials and Methods Patients were randomized 1:1 to receive palbociclib+ET (oral exemestane 25 mg/day for 28 days, palbociclib 125 mg/day for 21 days, plus leuprolide 3.75 mg subcutaneously every 4 weeks) or chemotherapy (oral capecitabine 1,250 mg/m2 twice daily for 14 days every 3 weeks). Tamoxifen resistance was defined as: relapse while on adjuvant tamoxifen, relapse within 12 months of completing adjuvant tamoxifen, or progression while on first-line tamoxifen within 6 months for MBC.
Results
In total, 184 patients were randomized and 178 were included in the modified intention-to-treat population. PFS improvement in the palbociclib+ET group was observed in tamoxifen-sensitive patients (hazard ratio, 0.38; 95% confidence interval, 0.12 to 1.19). Furthermore, palbociclib+ET prolonged median PFS compared with capecitabine in tamoxifen-sensitive (20.5 months vs. 12.6 months) and tamoxifen-resistant (20.1 months vs. 14.5 months) patients. Palbociclib+ET demonstrated a higher rate of objective response, disease control, and clinical benefit in tamoxifen-sensitive patients. Conclusion This post hoc exploratory analysis suggests that palbociclib+ET is a promising therapeutic option for premenopausal HR+/HER2– MBC patients irrespective of tamoxifen sensitivity.
Figure
Reference
-
References
1. Preusser M, De Mattos-Arruda L, Thill M, Criscitiello C, Bartsch R, Ruhstaller T, et al. CDK4/6 inhibitors in the treatment of patients with breast cancer: summary of a multidisciplinary round-table discussion. ESMO Open. 2018; 3:e000368.
Article2. Marra A, Curigliano G. Are all cyclin-dependent kinases 4/6 inhibitors created equal? NPJ Breast Cancer. 2019; 5:27.
Article3. Shah AN, Metzger O, Bartlett CH, Liu Y, Huang X, Cristofanilli M. Hormone receptor-positive/human epidermal growth receptor 2-negative metastatic breast cancer in young women: emerging data in the era of molecularly targeted agents. Oncologist. 2020; 25:e900–8.
Article4. Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016; 375:1925–36.
Article5. Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016; 17:425–39.
Article6. Park YH, Kim TY, Kim GM, Kang SY, Park IH, Kim JH, et al. Palbociclib plus exemestane with gonadotropin-releasing hormone agonist versus capecitabine in premenopausal women with hormone receptor-positive, HER2-negative metastatic breast cancer (KCSG-BR15-10): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2019; 20:1750–9.7. Kim JY, Kang D, Nam SJ, Kim SW, Lee JE, Yu JH, et al. Clinical features and outcomes of invasive breast cancer: age-specific analysis of a modern hospital-based registry. J Glob Oncol. 2019; 5:1–9.
Article8. Yeo W, Ueno T, Lin CH, Liu Q, Lee KH, Leung R, et al. Treating HR+/HER2− breast cancer in premenopausal Asian women: Asian Breast Cancer Cooperative Group 2019 Consensus and position on ovarian suppression. Breast Cancer Res Treat. 2019; 177:549–59.
Article9. Finn RS, Gelmon KA, Ettl J, Asselah J, Castrellon A, Ruiz Simón A, et al. Impact of prior treatment on palbociclib plus letrozole (P+L) efficacy and safety in patients (pts) with estrogen receptor-positive/human epidermal growth factor receptor 2-negative (ER+/HER2–) first-line advanced breast cancer (ABC): a PALOMA-2 subgroup analysis. Ann Oncol. 2017; 28(Suppl 5):v79–80.
Article10. Cardoso F, Senkus E, Costa A, Papadopoulos E, Aapro M, Andre F, et al. 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4). Ann Oncol. 2018; 29:1634–57.
Article11. Taylor CW, Green S, Dalton WS, Martino S, Rector D, Ingle JN, et al. Multicenter randomized clinical trial of goserelin versus surgical ovariectomy in premenopausal patients with receptor-positive metastatic breast cancer: an intergroup study. J Clin Oncol. 1998; 16:994–9.
Article12. Anders CK, Hsu DS, Broadwater G, Acharya CR, Foekens JA, Zhang Y, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008; 26:3324–30.
Article13. Liao S, Hartmaier RJ, McGuire KP, Puhalla SL, Luthra S, Chandran UR, et al. The molecular landscape of premenopausal breast cancer. Breast Cancer Res. 2015; 17:104.
Article14. Kan Z, Ding Y, Kim J, Jung HH, Chung W, Lal S, et al. Multi-omics profiling of younger Asian breast cancers reveals distinctive molecular signatures. Nat Commun. 2018; 9:1725.
Article15. Yap YS, Lu YS, Tamura K, Lee JE, Ko EY, Park YH, et al. Insights into breast cancer in the East vs the West: a review. JAMA Oncol. 2019; 5:1489–96.16. Lin CH, Yap YS, Lee KH, Im SA, Naito Y, Yeo W, et al. Contrasting epidemiology and clinicopathology of female breast cancer in Asians vs the US population. J Natl Cancer Inst. 2019; 111:1298–306.
Article17. Anders CK, Johnson R, Litton J, Phillips M, Bleyer A. Breast cancer before age 40 years. Semin Oncol. 2009; 36:237–49.
Article18. Park YH, Lee SJ, Jung HA, Kim SM, Kim MJ, Kil WH, et al. Prevalence and clinical outcomes of young breast cancer (YBC) patients according to intrinsic breast cancer subtypes: Single institutional experience in Korea. Breast. 2015; 24:213–7.
Article19. Ahn SH, Son BH, Kim SW, Kim SI, Jeong J, Ko SS, et al. Poor outcome of hormone receptor-positive breast cancer at very young age is due to tamoxifen resistance: nationwide survival data in Korea: a report from the Korean Breast Cancer Society. J Clin Oncol. 2007; 25:2360–8.20. Kim TY, Ahn JH, Yoon JH, Sohn JH, Kim GM, Lee KH, et al. Abstract P1-09-09: role of endocrine therapy in premenopausal patients with hormone receptor-positive metastatic breast cancer, compared with postmenopausal patients: diachronic analyses from nationwide cohort in Korea (KCSG BR 14-07). Cancer Res. 2016; 76(4 Suppl):P1-09-09.
Article21. Tripathy D, Im SA, Colleoni M, Franke F, Bardia A, Harbeck N, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018; 19:904–15.
Article22. Im SA, Lu YS, Bardia A, Harbeck N, Colleoni M, Franke F, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019; 381:307–16.
Article23. Turner NC, Slamon DJ, Ro J, Bondarenko I, Im SA, Masuda N, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018; 379:1926–36.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of the Efficacy between First-Line Treatment Regimens for Patients with Hormone Receptor-Positive and HER2-Negative Metastatic Breast Cancer
- Personalized therapy for advanced breast cancer using molecular signatures
- Hormone Treatment for Breast Cancer
- MicroRNA-222 Expression as a Predictive Marker for Tumor Progression in Hormone Receptor-Positive Breast Cancer
- Diagnosis and Treatment of HER2-Positive Breast Cancer