Endocrinol Metab.
2021 Jun;36(3):678-687. 10.3803/EnM.2021.978.
Heart Rate Variability in Postoperative Patients with Nonfunctioning Pituitary Adenoma
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Digital Healthcare Research Center, Taewoong Medical Co. Ltd., Gimpo, Korea
- 3Division of Endocrinology and Metabolism, Department of Internal Medicine, Yeouido St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2517658
- DOI: http://doi.org/10.3803/EnM.2021.978
Abstract
- Background
Decreased heart rate variability (HRV) has been reported to be associated with cardiac autonomic dysfunction. Hypopituitarism in nonfunctioning pituitary adenoma (NFPA) is often linked to increased cardiovascular mortality. We therefore hypothesized that postoperative NFPA patients with hormone deficiency have an elevated risk of HRV alterations indicating cardiac autonomic dysfunction.
Methods
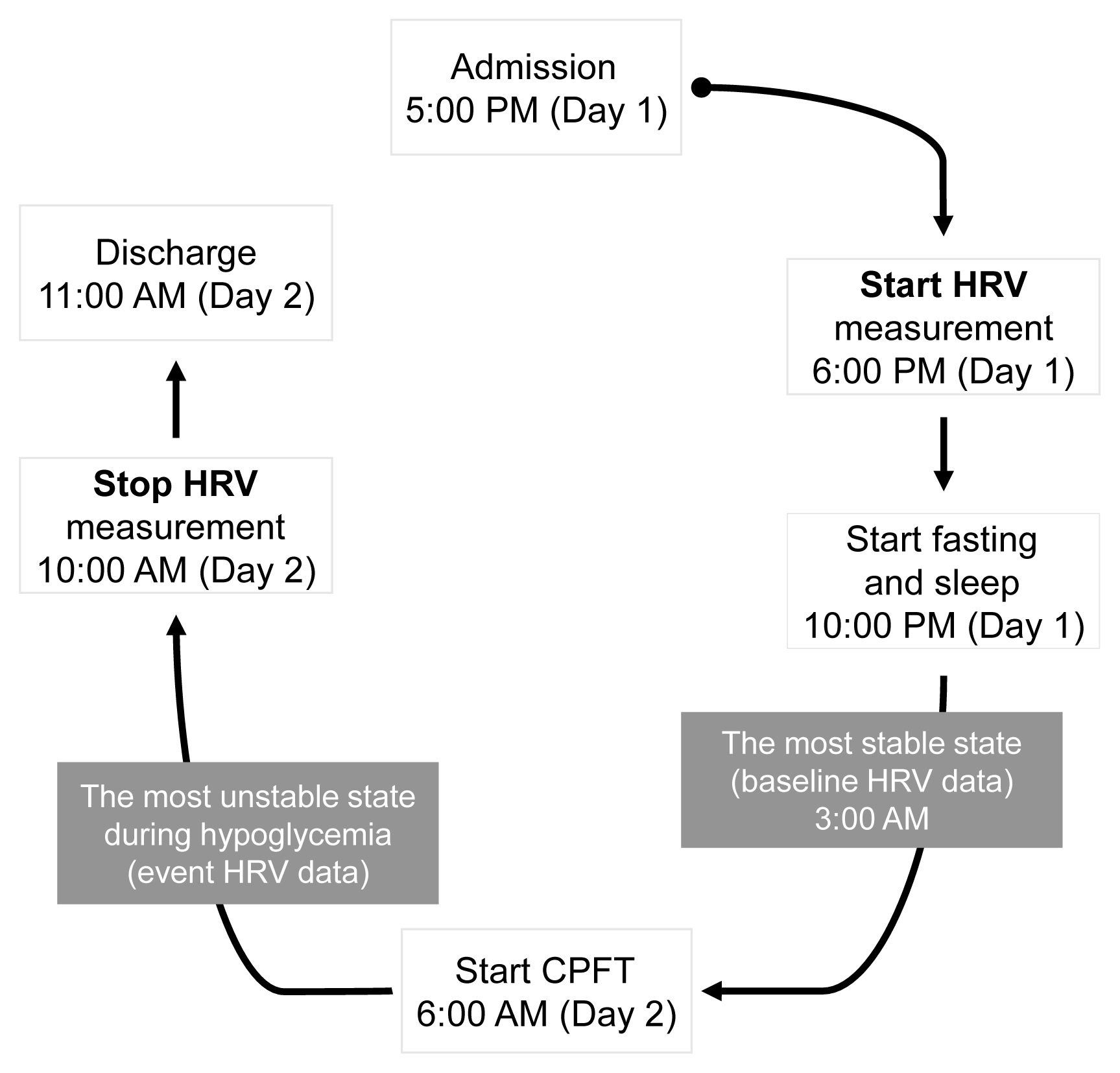
A total of 22 patients with NFPA were enrolled in the study. Between 3 and 6 months after surgery, a combined pituitary function test (CPFT) was performed, and HRV was measured. The period of sleep before the CPFT was deemed the most stable period, and the hypoglycemic period that occurred during the CPFT was defined as the most unstable period. Changes in HRV parameters in stable and unstable periods were observed and compared depending on the status of hormone deficiencies.
Results
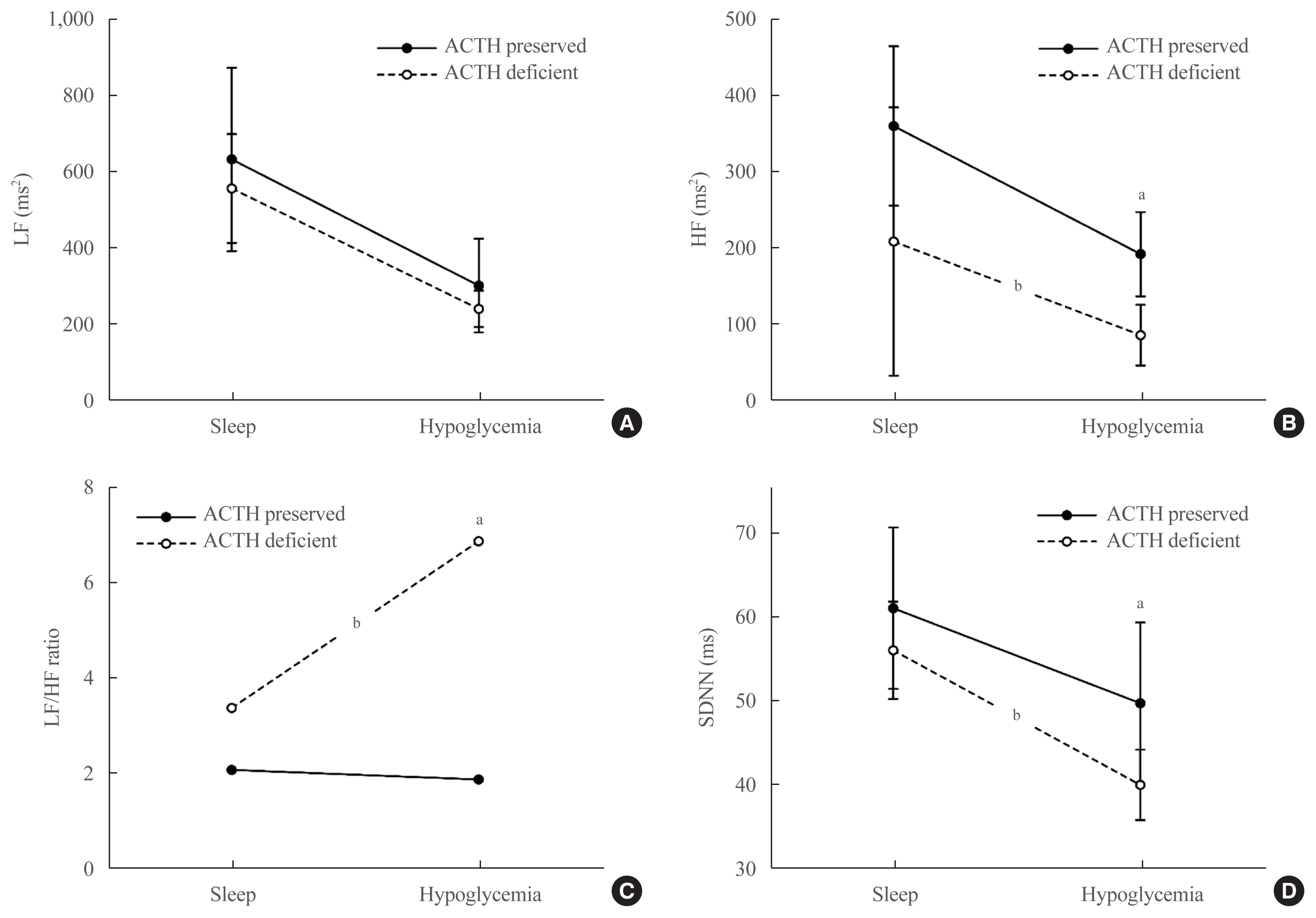
In patients with adrenocorticotropic hormone (ACTH) deficiency with other pituitary hormone deficiencies, the low frequency to high frequency ratio, which represents overall autonomic function and is increased in the disease state, was higher (P=0.005). Additionally, the standard deviation of the normal-to-normal interval, which decreases in the autonomic dysfunction state, was lower (P=0.030) during the hypoglycemic period. In panhypopituitarism, the low frequency to high frequency ratio during the hypoglycemic period was increased (P=0.007).
Conclusion
HRV analysis during CPFT enables estimation of cardiac autonomic dysfunction in patients with NFPA who develop ACTH deficiency with other pituitary hormone deficiencies or panhypopituitarism after surgery. These patients may require a preemptive assessment of cardiovascular risk.
Figure
Reference
-
1. Losa M, Mortini P, Barzaghi R, Ribotto P, Terreni MR, Marzoli SB, et al. Early results of surgery in patients with nonfunctioning pituitary adenoma and analysis of the risk of tumor recurrence. J Neurosurg. 2008; 108:525–32.
Article2. Rosen T, Bengtsson BA. Premature mortality due to cardiovascular disease in hypopituitarism. Lancet. 1990; 336:285–8.
Article3. Bates AS, Van’t Hoff W, Jones PJ, Clayton RN. The effect of hypopituitarism on life expectancy. J Clin Endocrinol Metab. 1996; 81:1169–72.
Article4. Erfurth EM, Bengtsson BA, Christiansen JS, Bulow B, Hagmar L. Premature mortality and hypopituitarism. Lancet. 2001; 357:1972.
Article5. Olsson DS, Nilsson AG, Bryngelsson IL, Trimpou P, Johannsson G, Andersson E. Excess mortality in women and young adults with nonfunctioning pituitary adenoma: a Swedish nationwide study. J Clin Endocrinol Metab. 2015; 100:2651–8.
Article6. Lindholm J, Nielsen EH, Bjerre P, Christiansen JS, Hagen C, Juul S, et al. Hypopituitarism and mortality in pituitary adenoma. Clin Endocrinol (Oxf). 2006; 65:51–8.
Article7. O’Reilly MW, Reulen RC, Gupta S, Thompson CA, Dineen R, Goulden EL, et al. ACTH and gonadotropin deficiencies predict mortality in patients treated for nonfunctioning pituitary adenoma: long-term follow-up of 519 patients in two large European centres. Clin Endocrinol (Oxf). 2016; 85:748–56.
Article8. Sherlock M, Reulen RC, Alonso AA, Ayuk J, Clayton RN, Sheppard MC, et al. ACTH deficiency, higher doses of hydrocortisone replacement, and radiotherapy are independent predictors of mortality in patients with acromegaly. J Clin Endocrinol Metab. 2009; 94:4216–23.
Article9. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996; 17:354–81.10. Rajendra Acharya U, Paul Joseph K, Kannathal N, Lim CM, Suri JS. Heart rate variability: a review. Med Biol Eng Comput. 2006; 44:1031–51.
Article11. Sim SY, Joo KM, Kim HB, Jang S, Kim B, Hong S, et al. Estimation of circadian body temperature rhythm based on heart rate in healthy, ambulatory subjects. IEEE J Biomed Health Inform. 2017; 21:407–15.
Article12. Kim HS, Yoon KH, Cho JH. Diurnal heart rate variability fluctuations in normal volunteers. J Diabetes Sci Technol. 2014; 8:431–3.
Article13. Park S, Kim WJ, Cho NJ, Choi CY, Heo NH, Gil HW, et al. Predicting intradialytic hypotension using heart rate variability. Sci Rep. 2019; 9:2574.
Article14. Tarvainen MP, Niskanen JP, Lipponen JA, Ranta-Aho PO, Karjalainen PA. Kubios HRV: heart rate variability analysis software. Comput Methods Programs Biomed. 2014; 113:210–20.15. Bilchick KC, Berger RD. Heart rate variability. J Cardiovasc Electrophysiol. 2006; 17:691–4.
Article16. Kim SY. Diagnosis and treatment of hypopituitarism. Endocrinol Metab (Seoul). 2015; 30:443–55.
Article17. Melmed S. Williams textbook of endocrinology. 14th ed. Philadelphia: Elsevier Saunders;2019. p. 184–235.18. Billman GE. Heart rate variability: a historical perspective. Front Physiol. 2011; 2:86.19. Cryer PE. Mechanisms of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med. 2013; 369:362–72.
Article20. Adler GK, Bonyhay I, Failing H, Waring E, Dotson S, Freeman R. Antecedent hypoglycemia impairs autonomic cardiovascular function: implications for rigorous glycemic control. Diabetes. 2009; 58:360–6.21. Soydan N, Bretzel RG, Fischer B, Wagenlehner F, Pilatz A, Linn T. Reduced capacity of heart rate regulation in response to mild hypoglycemia induced by glibenclamide and physical exercise in type 2 diabetes. Metabolism. 2013; 62:717–24.
Article22. Silva TP, Rolim LC, Sallum Filho C, Zimmermann LM, Malerbi F, Dib SA. Association between severity of hypoglycemia and loss of heart rate variability in patients with type 1 diabetes mellitus. Diabetes Metab Res Rev. 2017; 33:e2830.
Article23. De Ferrari GM, Sanzo A, Bertoletti A, Specchia G, Vanoli E, Schwartz PJ. Baroreflex sensitivity predicts long-term cardiovascular mortality after myocardial infarction even in patients with preserved left ventricular function. J Am Coll Cardiol. 2007; 50:2285–90.
Article24. Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007; 115:387–97.
Article25. Maser RE, Mitchell BD, Vinik AI, Freeman R. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta-analysis. Diabetes Care. 2003; 26:1895–901.26. Hassan-Smith ZK, Sherlock M, Reulen RC, Arlt W, Ayuk J, Toogood AA, et al. Outcome of Cushing’s disease following transsphenoidal surgery in a single center over 20 years. J Clin Endocrinol Metab. 2012; 97:1194–201.
Article27. Nilsson B, Gustavasson-Kadaka E, Bengtsson BA, Jonsson B. Pituitary adenomas in Sweden between 1958 and 1991: incidence, survival, and mortality. J Clin Endocrinol Metab. 2000; 85:1420–5.
Article28. Tomlinson JW, Holden N, Hills RK, Wheatley K, Clayton RN, Bates AS, et al. Association between premature mortality and hypopituitarism. West Midlands Prospective Hypopituitary Study Group. Lancet. 2001; 357:425–31.29. Hannon MJ, Crowley RK, Behan LA, O’Sullivan EP, O’Brien MM, Sherlock M, et al. Acute glucocorticoid deficiency and diabetes insipidus are common after acute traumatic brain injury and predict mortality. J Clin Endocrinol Metab. 2013; 98:3229–37.
Article30. Burman P, Mattsson AF, Johannsson G, Hoybye C, Holmer H, Dahlqvist P, et al. Deaths among adult patients with hypopituitarism: hypocortisolism during acute stress, and de novo malignant brain tumors contribute to an increased mortality. J Clin Endocrinol Metab. 2013; 98:1466–75.
Article31. Petersons CJ, Mangelsdorf BL, Thompson CH, Burt MG. Acute effect of increasing glucocorticoid replacement dose on cardiovascular risk and insulin sensitivity in patients with adrenocorticotrophin deficiency. J Clin Endocrinol Metab. 2014; 99:2269–76.
Article32. Zueger T, Kirchner P, Herren C, Fischli S, Zwahlen M, Christ E, et al. Glucocorticoid replacement and mortality in patients with nonfunctioning pituitary adenoma. J Clin Endocrinol Metab. 2012; 97:E1938–42.
Article33. Comunello A, Dassie F, Martini C, De Carlo E, Mioni R, Battocchio M, et al. Heart rate variability is reduced in acromegaly patients and improved by treatment with somatostatin analogues. Pituitary. 2015; 18:525–34.
Article34. Berntson GG, Bigger JT Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, et al. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997; 34:623–48.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pituitary Apoplexy due to Pituitary Adenoma Infarction
- Immunohistochemical Study on Pituitary Aednoma
- Developed diplopia and ptosis due to a nonfunctioning pituitary macroadenoma during pregnancy
- A Case of Impotence due to Pituitary Adenoma
- Two Cases of Pituitary Hyperplasia Secondary to Primary Hypothyroidism Mimicking Pituitary Tumor