Endocrinol Metab.
2021 Jun;36(3):514-524. 10.3803/EnM.2021.1082.
Current Guidelines for Management of Medullary Thyroid Carcinoma
- Affiliations
-
- 1Department of Internal Medicine, Biomedical Research Institute, Pusan National University Hospital, Busan, Korea
- KMID: 2517640
- DOI: http://doi.org/10.3803/EnM.2021.1082
Abstract
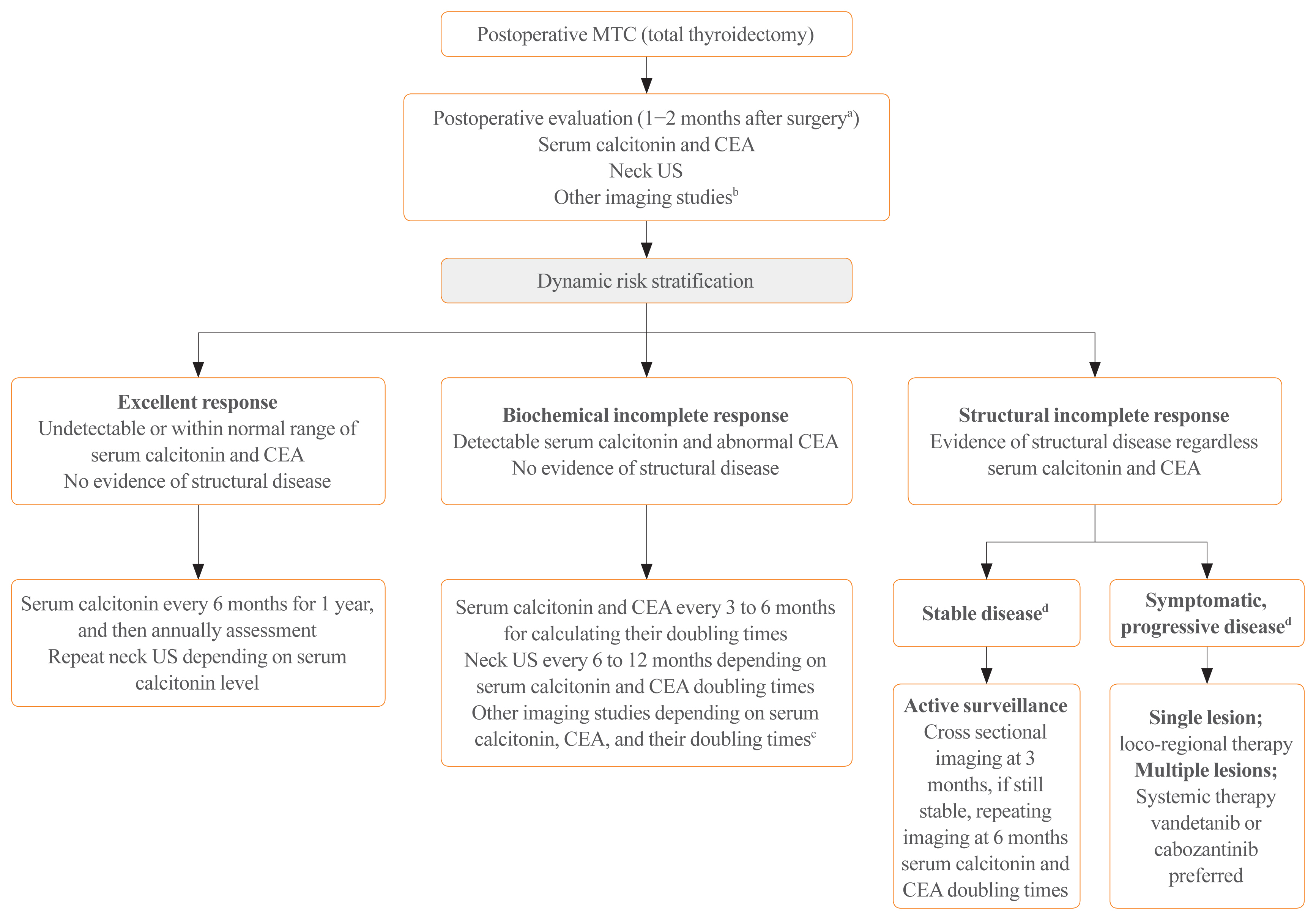
- Medullary thyroid carcinoma (MTC) is a rare neuroendocrine tumor originating from the parafollicular cells. The diagnostic and therapeutic strategies for the condition are different from those used for well-differentiated thyroid cancer. Since the 2015 American Thyroid Association guidelines for the diagnosis and treatment of MTC, the latest, including the National Comprehensive Cancer Network and European Association for Medical Oncology guidelines have been updated to reflect several recent advances in the management of MTC. Advances in molecular diagnosis and postoperative risk stratification systems have led to individualized treatment and follow-up strategies. Multi-kinase inhibitors, such as vandetanib and cabozantinib, can prolong disease progression-free survival with favorable adverse effects. In addition, potent selective rearranged during transfection (RET) inhibitors (selpercatinib and pralsetinib) have shown a promising efficacy in recent clinical trials. This review summarizes the management of MTC in recent guidelines focused on sporadic MTC.
Keyword
Figure
Cited by 1 articles
-
Distinct Impacts of Clinicopathological and Mutational Profiles on Long-Term Survival and Recurrence in Medullary Thyroid Carcinoma
Moon Young Oh, Kyong Yeun Jung, Hoonsung Choi, Young Jun Chai, Sun Wook Cho, Su-jin Kim, Kyu Eun Lee, Eun-Jae Chung, Do Joon Park, Young Joo Park, Han-Kwang Yang
Endocrinol Metab. 2024;39(6):877-890. doi: 10.3803/EnM.2024.2027.
Reference
-
1. Wells SA Jr, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015; 25:567–610.
Article2. Oh CM, Jung KW, Won YJ, Shin A, Kong HJ, Lee JS. Age-period-cohort analysis of thyroid cancer incidence in Korea. Cancer Res Treat. 2015; 47:362–9.
Article3. Pacini F, Castagna MG, Cipri C, Schlumberger M. Medullary thyroid carcinoma. Clin Oncol (R Coll Radiol). 2010; 22:475–85.
Article4. Filetti S, Durante C, Hartl D, Leboulleux S, Locati LD, Newbold K, et al. Thyroid cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019; 30:1856–83.
Article5. Ito Y, Onoda N, Okamoto T. The revised clinical practice guidelines on the management of thyroid tumors by the Japan Associations of Endocrine Surgeons: core questions and recommendations for treatments of thyroid cancer. Endocr J. 2020; 67:669–717.
Article6. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: thyroid carcinoma [Internet]. Plymouth Meeting: NCCN. c2021. [cited 2021 Jun 4]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf .7. Laure Giraudet A, Al Ghulzan A, Auperin A, Leboulleux S, Chehboun A, Troalen F, et al. Progression of medullary thyroid carcinoma: assessment with calcitonin and carcinoembryonic antigen doubling times. Eur J Endocrinol. 2008; 158:239–46.
Article8. Tran K, Khan S, Taghizadehasl M, Palazzo F, Frilling A, Todd JF, et al. Gallium-68 dotatate PET/CT is superior to other imaging modalities in the detection of medullary carcinoma of the thyroid in the presence of high serum calcitonin. Hell J Nucl Med. 2015; 18:19–24.9. Romei C, Casella F, Tacito A, Bottici V, Valerio L, Viola D, et al. New insights in the molecular signature of advanced medullary thyroid cancer: evidence of a bad outcome of cases with double RET mutations. J Med Genet. 2016; 53:729–34.
Article10. Machens A, Dralle H. Biomarker-based risk stratification for previously untreated medullary thyroid cancer. J Clin Endocrinol Metab. 2010; 95:2655–63.
Article11. Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al. 2017 AJCC cancer staging manual. 8th ed. New York: Springer;2017.12. Adam MA, Thomas S, Roman SA, Hyslop T, Sosa JA. Rethinking the current American Joint Committee on Cancer TNM staging system for medullary thyroid cancer. JAMA Surg. 2017; 152:869–76.
Article13. Stepanas AV, Samaan NA, Hill CS Jr, Hickey RC. Medullary thyroid carcinoma: importance of serial serum calcitonin measurement. Cancer. 1979; 43:825–37.
Article14. Lindsey SC, Ganly I, Palmer F, Tuttle RM. Response to initial therapy predicts clinical outcomes in medullary thyroid cancer. Thyroid. 2015; 25:242–9.
Article15. Kwon H, Kim WG, Jeon MJ, Song DE, Lee YM, Sung TY, et al. Dynamic risk stratification for medullary thyroid cancer according to the response to initial therapy. Endocrine. 2016; 53:174–81.
Article16. Krajewska J, Chmielik E, Jarzab B. Dynamic risk stratification in the follow-up of thyroid cancer: what is still to be discovered in 2017? Endocr Relat Cancer. 2017; 24:R387–402.
Article17. Yeh T, Yeung M, Sherman EJ, Tuttle RM, Sabra MM. Structural doubling time predicts overall survival in patients with medullary thyroid cancer in patients with rapidly progressive metastatic medullary thyroid cancer treated with molecular targeted therapies. Thyroid. 2020; 30:1112–9.
Article18. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–47.
Article19. Wells SA Jr, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012; 30:134–41.
Article20. Elisei R, Schlumberger MJ, Muller SP, Schoffski P, Brose MS, Shah MH, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013; 31:3639–46.
Article21. Kreissl MC, Bastholt L, Elisei R, Haddad R, Hauch O, Jarzab B, et al. Efficacy and safety of vandetanib in progressive and symptomatic medullary thyroid cancer: post hoc analysis from the ZETA Trial. J Clin Oncol. 2020; 38:2773–81.
Article22. Schlumberger M, Elisei R, Muller S, Schoffski P, Brose M, Shah M, et al. Overall survival analysis of EXAM, a phase III trial of cabozantinib in patients with radiographically progressive medullary thyroid carcinoma. Ann Oncol. 2017; 28:2813–9.
Article23. Kim M, Yoon JH, Ahn J, Jeon MJ, Kim HK, Lim DJ, et al. Vandetanib for the management of advanced medullary thyroid cancer: a real-world multicenter experience. Endocrinol Metab (Seoul). 2020; 35:587–94.
Article24. Koehler VF, Adam P, Frank-Raue K, Raue F, Berg E, Hoster E, et al. Real-world efficacy and safety of cabozantinib and vandetanib in advanced medullary thyroid cancer. Thyroid. 2021; 31:459–69.
Article25. Ramos HE, Hecht F, Berdelou A, Borget I, Leboulleux S, Baudin E, et al. Long-term follow-up and safety of vandetanib for advanced medullary thyroid cancer. Endocrine. 2021; 71:434–42.
Article26. Efstathiadou ZA, Tsentidis C, Bargiota A, Daraki V, Kotsa K, Ntali G, et al. Benefits and limitations of TKIs in patients with medullary thyroid cancer: a systematic review and meta-analysis. Eur Thyroid J. 2021; 10:125–39.
Article27. Ravaud A, de la Fouchardiere C, Caron P, Doussau A, Do Cao C, Asselineau J, et al. A multicenter phase II study of sunitinib in patients with locally advanced or metastatic differentiated, anaplastic or medullary thyroid carcinomas: mature data from the THYSU study. Eur J Cancer. 2017; 76:110–7.
Article28. Schlumberger M, Jarzab B, Cabanillas ME, Robinson B, Pacini F, Ball DW, et al. A phase II trial of the multitargeted tyrosine kinase inhibitor lenvatinib (E7080) in advanced medullary thyroid cancer. Clin Cancer Res. 2016; 22:44–53.
Article29. Hadoux J, Schlumberger M. Chemotherapy and tyrosine-kinase inhibitors for medullary thyroid cancer. Best Pract Res Clin Endocrinol Metab. 2017; 31:335–47.
Article30. Orlandi F, Caraci P, Berruti A, Puligheddu B, Pivano G, Dogliotti L, et al. Chemotherapy with dacarbazine and 5-fluorouracil in advanced medullary thyroid cancer. Ann Oncol. 1994; 5:763–5.
Article31. Li D, Chi Y, Chen X, Ge M, Zhang Y, Guo Z, et al. Anlotinib in locally advanced or metastatic medullary thyroid carcinoma: a randomized, double-blind phase IIB trial. Clin Cancer Res. 2021. Apr. 8. [Epub]. https://doi.org/10.1158/1078-0432.CCR-20-2950 .
Article32. De Falco V, Buonocore P, Muthu M, Torregrossa L, Basolo F, Billaud M, et al. Ponatinib (AP24534) is a novel potent inhibitor of oncogenic RET mutants associated with thyroid cancer. J Clin Endocrinol Metab. 2013; 98:E811–9.
Article33. Drilon AE, Zhai D, Rogers E, Deng W, Zhang X, Ung J, et al. The next-generation RET inhibitor TPX-0046 is active in drug-resistant and naïve RET-driven cancer models. J Clin Oncol. 2020; 38(15 Suppl):3616.
Article34. Wirth LJ, Sherman E, Robinson B, Solomon B, Kang H, Lorch J, et al. Efficacy of selpercatinib in RET-altered thyroid cancers. N Engl J Med. 2020; 383:825–35.
Article35. Bradford D, Larkins E, Mushti SL, Rodriguez L, Skinner AM, Helms WS, et al. FDA approval summary: selpercatinib for the treatment of lung and thyroid cancers with RET gene mutations or fusions. Clin Cancer Res. 2021; 27:2130–5.36. Subbiah V, Hu MI, Ganior JF, Mansfield AS, Alonso G, Taylor MH, et al. Clinical activity of the RET inhibitor pralsetinib (BLU-667) in patients with RET fusion+ solid tumors. J Clin Oncol. 2020; 38(15 Suppl):109.
Article37. Markham A. Pralsetinib: first approval. Drugs. 2020; 80:1865–70.
Article38. Drilon A, Rogers E, Zhai D, Deng W, Zhang X, Lee D, et al. 506P: TPX-0046 is a novel and potent RET/SRC inhibitor for RET-driven cancers. Ann Oncol. 2019; 30(Suppl 5):v190–1.39. Schoffski P, Aftimos PG, Massard C, Italiano A, Jungels C, Andreas K, et al. A phase I study of BOS172738 in patients with advanced solid tumors with RET gene alterations including non-small cell lung cancer and medullary thyroid cancer. J Clin Oncol. 2019; 37(15 Suppl):TPS3162.
Article40. Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020; 21:1353–65.
Article41. Araque KA, Gubbi S, Klubo-Gwiezdzinska J. Updates on the management of thyroid cancer. Horm Metab Res. 2020; 52:562–77.
Article42. Stift A, Sachet M, Yagubian R, Bittermann C, Dubsky P, Brostjan C, et al. Dendritic cell vaccination in medullary thyroid carcinoma. Clin Cancer Res. 2004; 10:2944–53.
Article43. Papewalis C, Wuttke M, Jacobs B, Domberg J, Willenberg H, Baehring T, et al. Dendritic cell vaccination induces tumor epitope-specific Th1 immune response in medullary thyroid carcinoma. Horm Metab Res. 2008; 40:108–16.
Article44. Kraeber-Bodere F, Rousseau C, Bodet-Milin C, Ferrer L, Faivre-Chauvet A, Campion L, et al. Targeting, toxicity, and efficacy of 2-step, pretargeted radioimmunotherapy using a chimeric bispecific antibody and 131I-labeled bivalent hapten in a phase I optimization clinical trial. J Nucl Med. 2006; 47:247–55.45. Chatal JF, Campion L, Kraeber-Bodere F, Bardet S, Vuillez JP, Charbonnel B, et al. Survival improvement in patients with medullary thyroid carcinoma who undergo pretargeted anti-carcinoembryonic-antigen radioimmunotherapy: a collaborative study with the French Endocrine Tumor Group. J Clin Oncol. 2006; 24:1705–11.
Article46. Salavati A, Puranik A, Kulkarni HR, Budiawan H, Baum RP. Peptide receptor radionuclide therapy (PRRT) of medullary and nonmedullary thyroid cancer using radiolabeled somatostatin analogues. Semin Nucl Med. 2016; 46:215–24.
Article47. Iten F, Muller B, Schindler C, Rochlitz C, Oertli D, Macke HR, et al. Response to [90Yttrium-DOTA]: TOC treatment is associated with long-term survival benefit in metastasized medullary thyroid cancer. A phase II clinical trial. Clin Cancer Res. 2007; 13(22 Pt 1):6696–702.48. Beukhof CM, Brabander T, van Nederveen FH, van Velthuysen MF, de Rijke YB, Hofland LJ, et al. Peptide receptor radionuclide therapy in patients with medullary thyroid carcinoma: predictors and pitfalls. BMC Cancer. 2019; 19:325.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Concurrent Medullay and Papillary Carcinoma of the Thyroid
- Medullary Thyroid Carcinoma with Normal Calcitonin Level
- Concurrent Papillary and Medullary Carcinoma of the Thyroid Gland
- A Case of Concurrent Papillary and Medullary Thyroid Carcinomas Detected as Recurrent Medullary Carcinoma after Initial Surgery for Papillary Carcinoma
- Medullary and Papillary Thyroid Carcinoma as a Collision Tumor: Report of Five Cases